Chemical Properties
Garnet-red prisms with metallic luster.
Soluble in chloroform, benzene, and
pyridine; slightly soluble in alcohol and methanol.
Uses
A natural caratenoid pigment that is converted to Vitamin A in the human body. It is an antioxidant and may help prevent free radical damage to cells and DNA, as well as stimulate the repair of oxidative damage to DNA. A potential chemopreventative agent against lung cancer as well as other types of cancer.
Uses
β-Cryptoxanthin has also been used to study its effect on the production of immunoglobulins in Peyer′s patch cells
ex- vivo. It has also been used as a standard in high-performance liquid chromatography (HPLC analysis).(5)
Definition
ChEBI: A carotenol that exhibits antioxidant activity. It has been isolated from fruits such as papaya and oranges.
General Description
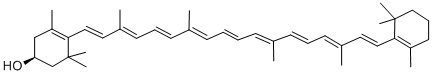
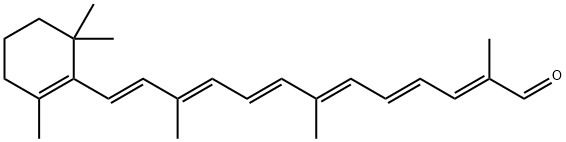
β-Cryptoxanthin is an oxygen-containing carotenoid and is a member of the xanthophyll family. It is one of the major sources of vitamin A and is present in fruits such as oranges, tangerines and papayas. In addition, it is also found in corn, peas and some animal products such as egg yolk and butter.
Biochem/physiol Actions
β-Cryptoxanthin exhibits?potential-anabolic effect on bone calcification?by stimulating osteoblastic bone formation and inhibiting osteoclastic bone resorption in vitro. It acts as an antioxidant and avoids free radical damage to biomolecules such as lipids, proteins and nucleic acids. High dietary intake of β-cryptoxanthin reduces the risk of developing rheumatoid arthritis and lung cancer.
Purification Methods
Purify it by chromatography on MgO, CaCO3 or deactivated alumina, using EtOH or diethyl ether to develop the column. Crystallise it from *C6H6/EtOH (metallic prisms), or needles from *C6H6. Store it in the dark under N2 or Ar at -20o. The acetate has m 117.5o. The racemate is purified through a column of alumina (grade IV), eluted with *C6H6 then EtOAc/*C6H6 (1:9) and recrystallised from pet ether (b 60-80o) with m 172-173o. [Loeber et al. J Chem Soc (C) 404 1971, Goodfellow et al. J Chem Soc Chem Commun 1578 1970, Isler et al. Helv Chim Acta 40 456 1957, Beilstein 6 III 3772, 6 IV 5111.]