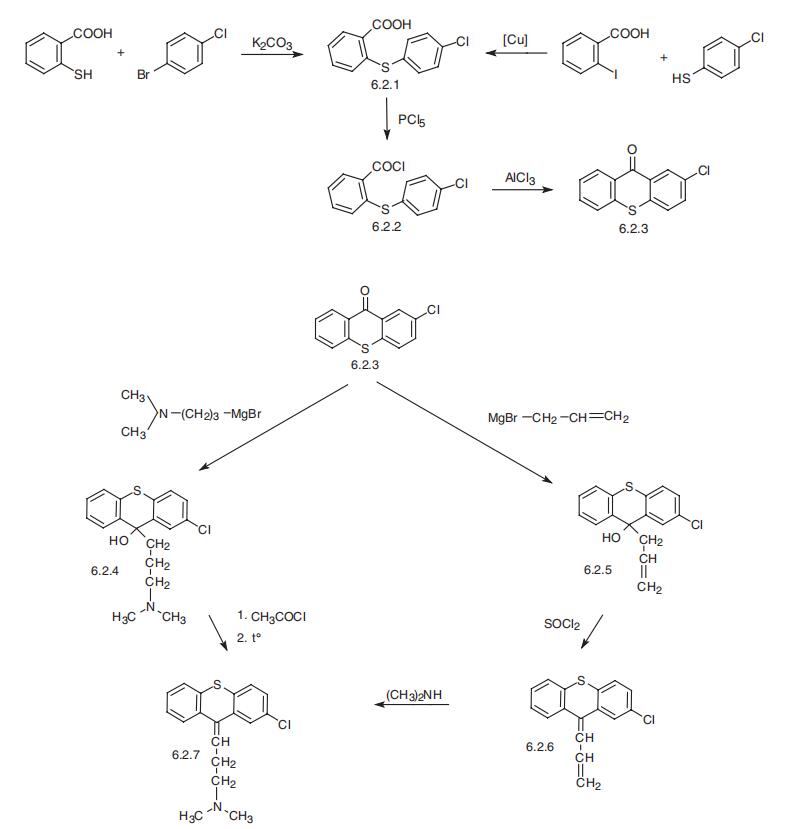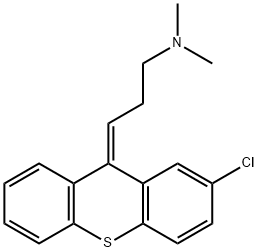
Chlorprothixene
- Product NameChlorprothixene
- CAS113-59-7
- CBNumberCB6361448
- MFC18H18ClNS
- MW315.86
- EINECS204-032-8
- MDL NumberMFCD00869180
- MOL File113-59-7.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 97-98° |
| Boiling point | 160 °C(Press: 0.04 Torr) |
| Density | 1.1048 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO : 33.33 mg/mL (105.52 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) |
| form | powder to crystal |
| pka | pKa 8.4(H2O) (Uncertain) |
| color | White to Orange to Green |
| Water Solubility | 385.8ug/L(22.5 ºC) |
| Stability | Hygroscopic |
| FDA UNII | 9S7OD60EWP |
| ATC code | N05AF03 |
| NIST Chemistry Reference | Chlorprothixene(113-59-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301-H336 | |||||||||
| Precautionary statements | P301+P310+P330 | |||||||||
| HS Code | 2934.99.3000 | |||||||||
| Hazardous Substances Data | 113-59-7(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in rabbit: 182mg/kg | |||||||||
| NFPA 704: |
|