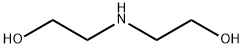
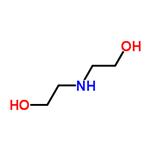
Description
Diethanolamine is an organic base which has been used as an emulsifying and dispersing agent.It can also be used as a basic buffer, with optimal pH about pH 9, if titrated with HCl or other acid. Other uses include: to "scrub" gases, as a chemical intermediate, as humectant or softening agent.
Chemical Properties
The USP32–NF27 describes diethanolamine as a mixture of ethanolamines consisting largely of diethanolamine. At about room temperature it is a white, deliquescent solid. Above room temperature diethanolamine is a clear, viscous liquid with a mildly ammoniacal odor.

Diethanolamine is used as surface-active agent in metal-cutting fluids and oils, as a corrosion inhibitor, as a dispersant in agricultural chemical formulations, and as an intermediate in the production of other compounds such as fatty acid condensates of diethanolamine which are extensively used in soaps and cosmetics as emulsifiers, thickeners, wetting agents and detergents (Beyer et al., 1983). In the cosmetic formulations, the concentration of diethanolamine may range from 1 to 25% (National Toxicology Program, 1999a).
Uses
Diethanolamine similar to triethanolamine (T775580) is used as a surfactant. It also has the potential to be a corrosion inhibitor by means of chemisorption.
Uses
To scrub gases as indicated under ethanolamine. Diethanolamine can be used with cracking gases and coal or oil gases which contain carbonyl sulfide that would react with monoethanolamine. As rubber chemicals intermediate. In the manufacture of surface active agents used in textile specialties, herbicides, petroleum demulsifiers. As emulsifier and dispersing agent in various agricultural chemicals, cosmetics, and pharmaceuticals. In the production of lubricants for the textile industry. As humectant and softening agent. In organic syntheses.
Uses
Diethanolamine is used in the production ofsurface-active agents and lubricants for thetextile industry; as an intermediate for rubberchemicals; as an emulsifier; as a humectantand softening agent; as a detergent in paints,shampoos, and other cleaners; and as anintermediate in resins and plasticizers.
Preparation
Diethanolamine is prepared commercially by the ammonolysis of ethylene oxide. The reaction yields a mixture of monoethanolamine, diethanolamine, and triethanolamine which is separated to obtain the pure products.
Production Methods
Diethanolamine is produced with monoethanolamine and triethanolamine by
ammonolysis of ethylene oxide; diethanolamine is then separated by distillation
(Mullins 1978). In 1984, 166.2 million pounds of diethanolamine were produced
in the United States (USTIC 1985).
Definition
ChEBI: A member of the class of ethanolamines that is ethanolamine having a N-hydroxyethyl substituent.
General Description
Oily colorless liquid or solid white crystals. Slight rotten fish or ammonia odor. Denser than water.
Air & Water Reactions
Water soluble.
Reactivity Profile
2,2'-Iminodiethanol is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. 2,2'-Iminodiethanol is hygroscopic. 2,2'-Iminodiethanol may be sensitive to exposure to air and light. 2,2'-Iminodiethanol can react with oxidizing materials, acids, CO2, copper alloys, aluminum, zinc, galvanized iron and copper.
Health Hazard
The irritant action of diethanolamine on theeyes can be severe. Direct contact of thepure liquid can impair vision. Irritation onthe skin may be mild to moderate. Theacute oral toxicity of this compound waslow in test animals. The toxic symptomsinclude somnolence, excitement, and musclecontraction.
LD50 value, oral (mice): 3300 mg/kg
The vapor pressure of diethanolamine isnegligibly low (<0.01 torr at 20°C (68°F)).At ordinary temperature, this compoundshould not cause any inhalation hazard. Themists, fumes, or vapors at high temperatures,however, can produce eye, skin, and respiratory tract irritation.
In contrast to monoethanolamine, dieth anolamine administered to mice at 1125 mg/kg/day caused no change in maternal mortality, litter size, or percentage survival of thepups (Environmental Health Research andTesting 1987).
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors are generated when heated.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water : No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Pharmaceutical Applications
Diethanolamine is primarily used in pharmaceutical formulations as a buffering agent, such as in the preparation of emulsions with fatty acids. In cosmetics and pharmaceuticals it is used as a pH adjuster and dispersant.
Diethanolamine has also been used to form the soluble salts of active compounds, such as iodinated organic acids that are used as contrast media. As a stabilizing agent, diethanolamine prevents the discoloration of aqueous formulations containing hexamethylenetetramine-1,3-dichloropropene salts.
Diethanolamine is also used in cosmetics.
Industrial uses
Diethanolamine undergoes reactions characteristic of secondary amines and of
alcohols. Two industrially important reactions of the ethanolamines involve
reaction with carbon dioxide or hydrogen sulfide to yield water soluble salts, and
reaction with long chain fatty acids to form neutral ethanolamine soaps (Mullins
1978). Substituted ethanolamine compounds, such as soaps, are used extensively
as emulsifiers, thickeners, wetting agents, and detergents in cosmetic formulations
(including skin cleaners, creams, and lotions) (Beyer et al 1983).
Diethanolamine is used as a dispersing agent in various agricultural chemicals,
as an absorbent for acidic gases (hydrogen sulfide and carbon dioxide), as a
humectant, as an intermediate in the synthesis of morpholine, as a surface-active
agent in cutting fluids, as a corrosion inhibitor, as a component in textile specialty
agents, and as a secondary vulcanization accelerator in the rubber industry.
Diethanolamine is also used in cleaners and pharmaceutical ointments, in polyurethane
formulations, in herbicides, and in a variety of organic syntheses (Beyer
et al 1983; Mullins 1978; Windholz 1983). Diethanolamine is permitted in articles
intended for use in the production, processing, or packaging of food (CFR 1981),
and is permitted as a secondary direct food additive from use in delinting
cottonseed in the production of cottonseed oil or meal cake (Fed. Reg. 1982).
Because of the wide industrial and consumer uses, large amounts of this chemical
are discharged into water and sewage in an unaltered form (Yordy and Alexander
1981).
Contact allergens
Diethanolamine is contained in many products, as a
metalworking fuid. Traces may exist in other etha-
nolamine-containing fuids.
Safety Profile
Poison by
intraperitoneal route. Moderately toxic by
ingestion and subcutaneous routes. Mildly
toxic by skin contact. A severe eye and mild
skin irritant. Experimental reproductive
effects. Combustible when exposed to heat
or flame; can react with oxidizing materials.
To fight fire, use alcohol foam, water, Co2,
dry chemical. When heated to
decomposition it emits toxic fumes such as
NOx. See also AMINES.
Safety
Diethanolamine is used in topical and parenteral pharmaceutical formulations, with up to 1.5% w/v being used in intravenous infusions. Experimental studies in dogs have shown that intravenous administration of larger doses of diethanolamine results in sedation, coma, and death.
Animal toxicity studies suggest that diethanolamine is less toxic than monoethanolamine, although in rats the oral acute and subacute toxicity is greater. Diethanolamine is said to be heptacarcinogenic in mice and has also been reported to induce hepatic choline deficiency in mice.
Diethanolamine is an irritant to the skin, eyes, and mucous membranes when used undiluted or in high concentration. However, in rabbits, aqueous solutions containing 10% w/v diethanolamine produce minor irritation. The lethal human oral dose of diethanolamine is estimated to be 5–15g/kg body-weight.
The US Cosmetic Ingredient Review Expert Panel evaluated diethanolamine and concluded that it is safe for use in cosmetic formulations designed for discontinuous, brief use followed by thorough rinsing from the surface of the skin. In products intended for prolonged contact with the skin, the concentration of ethanolamines should not exceed 5%. Diethanolamine should not be used in products containing N-nitrosating agents.
LD50 (guinea pig, oral): 2.0g/kg
LD50 (mouse, IP): 2.3g/kg
LD50 (mouse, oral): 3.3g/kg
LD50 (rabbit, skin): 12.2g/kg
LD50 (rat, IM): 1.5g/kg
LD50 (rat, IP): 0.12g/kg
LD50 (rat, IV): 0.78g/kg
LD50 (rat, oral): 0.71g/kg
LD50 (rat, SC): 2.2g/kg
Potential Exposure
Diethanolamine is present in machining and grinding fluids and has been detected in workplace air in the metal manufacturing industry. It was present in bulk cutting fluids at levels ranging from 4 to 5% (Kenyon et al., 1993). Diethanolamine has also been reported to be present in wetting fluids used in road paving. A level of 0.05 mg/m3 was detected in a stationary sample at a slurry machine discharging a bitumen emulsion containing 0.2% of the amine. All personal exposures were below the detection limit (0.02 mg/m3) (Levin et al., 1994). In a German study (1992–94), diethanolamine was measured in samples of metalworking fluids in a range of 0–44% (n = 69). The number of samples with diethanolamine present steadily declined from 90% to 60% over the study period (Pfeiffer et al., 1996).
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
When DEA was administered cutaneously
to pregnant rats and rabbits during organogenesis,
developmental toxicity (skeletal variations)
was observed only in the rat and only at
doses causing significant maternal toxicity.
The 2003 ACGIH threshold limit valuetime-
weighted average (TLV-TWA) is 3ppm
(13mg/m3).
Metabolism
Treatment of Wistar or Sherman rats with diethanolamine caused increases in the
formation of hepatic phospholipids (Artom et al 1949). In addition, dietary
administration led to incorporation of ethanolamine into hepatic phospholipids
(Artom et al 1949), and repeated oral administration of diethanolamine in drinking
water (one to three wk) at a dose of 320 mg/kg/d was found to reduce the level of
incorporation of ethanolamine and choline into hepatic and renal phospholipids in
Sprague-Dawley rats (Barbee and H?rtung 1979b).
Dermal absorption of diethanolamine is suggested to occur in rats since Nnitrosodiethanolamine
was excreted in the urine of male Sprague-Dawley rats
which had been administered diethanolamine by dermal application and given
nitrite in their drinking water (Preussman et al 1981).
storage
Diethanolamine is hygroscopic and light- and oxygen-sensitive; it should be stored in an airtight container, protected from light, in a cool, dry place.
Shipping
UN2491 Ethanol
amine or Ethanolamine solutions, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Fractionally distil the amine twice, then fractionally crystallise it from its melt. Its solubility in H2O is 10% at 20o. [Perrin & Dempsey Buffers for pH and Metal Ion Control Chapman & Hall, London 1974, Beilstein 4 H 283, 4 II 729, 4 III 689, 4 IV 1514.]
Incompatibilities
Diethanolamine is a secondary amine that contains two hydroxy groups. It is capable of undergoing reactions typical of secondary amines and alcohols. The amine group usually exhibits the greater activity whenever it is possible for a reaction to take place at either the amine or a hydroxy group.
Diethanolamine will react with acids, acid anhydrides, acid chlorides, and esters to form amide derivatives, and with propylene carbonate or other cyclic carbonates to give the corresponding carbonates. As a secondary amine, diethanolamine reacts with aldehydes and ketones to yield aldimines and ketimines. Diethanolamine also reacts with copper to form complex salts. Discoloration and precipitation will take place in the presence of salts of heavy metals.
Toxics Screening Level
The initial threshold screening level (ITSL) for diethanolamine (DEA) is 0.2 μg/m3 (annual averaging time) and an acute ITSL for diethanolamine is 10 μg/m3 (8-hour averaging time).
Waste Disposal
Controlled incineration; incinerator equipped with a scrubber or thermal unit to reduce
nitrogen oxides emissions
Regulatory Status
Included in the FDA Inactive Ingredients Database (IV infusions, ophthalmic solutions, and topical preparations). Included in medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.