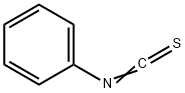
Chemical Properties
colourless to pale yellow liquid with a penetrating odour
Uses
Phenyl isothiocyanate is the reagent of choice in automated Edman degradation systems. However, this reagent is highly toxic and for manual modification other reagents are preferred such as dimethy laminoazobenzene isothiocyanate (Chang, 1983; Wang et al, 2000) or trifluoroethyl isothiocyanate (Bartlet-Jones et al, 1994). This last reagent has the advantage of being volatile, so that the excess of reagent is easily removed by vacuum before MS analysis (Spengler, 1997). This compound was used successfully during seven successive cycles of manual cleavage coupled with MS analysis. Nevertheless, the high reactivity of such compounds seems to shows artefactual modification such as“acetylation' of the hydroxyl groups of the Ser and Thr. Allyl isothiocyanate shows better selectivity, but again this reagent is toxic and difficult to use in manual approaches (Gu & Preswich, 1997) .
Uses
Phenyl isothiocyanate acts as a derivatizing reagent for primary and secondary amines. It is used in sequencing peptides by Edman degradation and in amino acid analyses by HPLC. It is used for derivatizing N-terminal amino acids of proteins for automated sequential analysis. It is a synthon for dithiadiazafulvalenes.
Preparation
Synthetic procedure for phenyl isothiocyanate in 1-mol scale
Into a 2-L jacketed flask, 91.2 g of CS2 (1.2 mol) was dropwise added to a mixture of aniline (93.0 g, 1.0 mol) and K2CO3 (276.0 g, 2.0 mol) in 700 mL of water at room temperature within a period of 2.5 h. After the addition was complete, the mixture was stirred another 2 h. Then, the mixture was cooled to 0 °C, and a solution of 92.3 g of TCT (0.5 mol) in 450 mL of CH2Cl2 was dropwise added within 4 h. After the addition was complete, the mixture was stirred another 1 h to complete the conversion. The resulting mixture was basified to pH >11 with 250 mL of 6 N NaOH and yielded a clear solution. The organic layer was separated and the aqueous layer was extracted with 150 mL of CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4, filtered, and the solvent of filtrate was removed via distillation under atmospheric pressure with a 25-cm Vigreux column. The residue was vacuum distilled and the desired product fraction was collected at 72–74 °C/1 mmHg. Finally, 127.0 g of colorless liquid (94%) was obtained.
Definition
ChEBI: Phenyl isothiocyanate is an isothiocyanate having a phenyl group attached to the nitrogen; used for amino acid sequencing in the Edman degradation. It has a role as an allergen and a reagent.
Biological Functions
Phenyl isothiocyanate (PITC) is a well-established reagent in protein chemistry since its introduction in Edman degradation. PITC reacts with primary and secondary amines under alkaline conditions within 20 min. The resulting phenylthiocarbamyl (PTC) derivatives of the amino acids are stable and do not interfere with reaction by-products during chromatography. The absorption maximum is around 245 nm with a detection limit of 1 pmol.
Phenyl isothiocyanate may be employed as a derivatization reagent for high-performance liquid chromatographic (HPLC) analysis of various amphetamine derivatives in body fluids for forensic purposes.
General Description
Phenyl isothiocyanate is an aromatic isothiocyanate. It participates in dehydration reactions of alcohols. It is widely used for synthesis of various biologically important heterocyclic compounds.
Purification Methods
It is insoluble in H2O, but soluble in Et2O and EtOH. If impure (due to formation of thiourea), then steam distil it into a receiver containing 5-10mL of N H2SO4. Separate the oil, dry over CaCl2 and distil it under vacuum. [Dains et al. Org Synth Coll Vol I 447 1941, Beilstein 12 IV 867.]