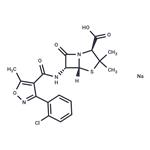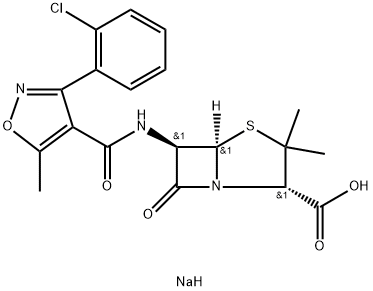
Cloxacillin-13C4 SodiuM Salt
- Product NameCloxacillin-13C4 SodiuM Salt
- CAS642-78-4
- CBNumberCB5705412
- MFC19H19ClN3NaO5S
- MW459.88
- EINECS211-390-9
- MDL NumberMFCD00150735
- MOL File642-78-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 170°C |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water |
| BCS Class | 3 |
| Stability | Stable. Incompatible with strong oxidizing agents. Refrigerate. |
| CAS DataBase Reference | 642-78-4(CAS DataBase Reference) |
| FDA UNII | MWQ645MKMF |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H317-H334 | |||||||||
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 36/37/38-42/43 | |||||||||
| Safety Statements | 26-36-22 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XH8920000 | |||||||||
| NFPA 704: |
|