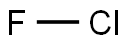Chemical Properties
colorless gas; slightly yellow when liquid; enthalpy of vaporization 24 kJ/mol; specific conductivity 1.9×10?7 ohm· cm; destroys glass instantly, attacks quartz readily in presence of moisture; organic matter bursts into flame instantly on contact [MER06] [CRC10]
Synthesis
A nickel or Monel cylinder within a furnace is used as a reaction vessel where chlorine and fluorine gases are introduced. The setup includes a condenser and traps to condense and separate the gases, with ClF being liquefied in a trap cooled by liquid nitrogen. The process involves heating to 400°C, adjusting the flow rate of chlorine for a given fluorine cell current, and collecting the product in a cooled trap. After fluorination, ClF is distilled into a steel cylinder, with the system pressure maintained at about 1 atm. The distillation stops when condensate accumulates in a quartz trap, and the yield is up to 90% based on chlorine.