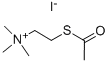Description
Acetylthiocholine iodide, also known as ATCI, serves as both an acetylcholinesterase (AChE) substrate and a nicotinic acetylcholine receptor (AChR) agonist. ATCI itself is a colorless and odorless compound. It exhibits solubility in both water and ethanol. It is a synthetic compound extensively employed in laboratory experiments to measure the activity of AChE, a vital enzyme responsible for the breakdown of the neurotransmitter acetylcholine (ACh) in the central and peripheral nervous systems. Additionally, it enables the evaluation of drug effects on AChE activity and facilitates investigations into the impact of toxins on the nervous system. Acetylthiocholine iodide functions as an inhibitor of AChE. By binding to the enzyme′s active site, it prevents AChE from catalyzing the breakdown of ACh. Consequently, ACh accumulates in the synaptic cleft, leading to increased neuron excitability. This inhibition of AChE by ATCI ultimately results in a higher ACh concentration within the synaptic cleft, yielding a range of physiological effects. These effects encompass heightened muscle contraction, increased heart rate and blood pressure, elevated salivation, intensified bronchial secretions, and augmented gastrointestinal activity[1].
Chemical Properties
white crystalline powder
Uses
Substrate for the colorimetric determination of acetylcholinesterase activity.
Uses
Acetylthiocholine iodide has been used as a substrate in the preparation of acetylcholine esterase (AChE) assay working solution for AChE activity assay. It has also been used as a component in phosphate buffer (PB) for cholinesterase assay.
Uses
A nAChR agonist and substrate of acetylcholinesterase
Purification Methods
Recrystallise the iodide from propan-1-ol (or iso-PrOH, or EtOH/Et2O) until almost colourless and dry it in a vacuum desiccator over P2O5. Its solubility in H2O is 1% w/v. A 0.075M (21.7mg/mL) solution in 0.1M phosphate buffer pH 8.0 is stable for 10-15 days if kept refrigerated. Store it away from light. It is commercially available as a 1% solution in H2O. [Ellman et al. Biochemical Pharmacology 7, 88 1961, IR: Hansen Acta Chem Scand 13 151 1959, 11 537 1957, Clin Chim Acta 2 316 1957, Ivin Zh Obshch Khim 22 267 1952, Beilstein 4 III 726, 4 IV 1585.]
References
[1] Madalina-Petruta Bucur, Gabriel-Lucian Radu, Bogdan Bucur. “Critical evaluation of acetylthiocholine iodide and acetylthiocholine chloride as substrates for amperometric biosensors based on acetylcholinesterase.” Sensors 13 2 (2013): 1603–13.