Manufacturing Process
42.4 g (0.2 mol) of (4-fluorophenyl)-pyrid-2-yl-acetonitrile are dissolved in 50
ml of dimethylformamide and introduced dropwise into a suspension, cooled
with ice, of 5.0 g of sodium hydride (put into the process as a dispersion in
mineral oil) in 150 ml of dimethylformamide. The reaction mixture is then
stirred at room temperature for 15 min and thereafter heated under reflux for
5 h after the addition of 53.4 g (0.21 mol) of N-(2-bromoethyl)phthalimide.
When the resulting reaction mixture has cooled down, it is diluted with 500 ml
of ether and the organic phase is washed with water until neutral, dehydrated
over sodium sulfate and then concentrated by evaporation under vacuum. The
N-[3-(cyano-3-(4-fluorophenyl)-3-(pyrid-2-yl)propyl]phthalimide, oily residue,
melting point 154°C (crystallizing from methanol), yield: 48.5 g (63%).
46.25 g (0.12 mol) of N-[3-cyano-3-(4-fluorophenyl)-3-(pyrid-2-
yl)propyl]phthalimide in 100 ml of 75% sulfuric acid are heated to 150°C for 5
h. When the reaction mixture is cold, it is poured out on ice, filtered through a
glass filter, alkalized with sodium hydroxide solution and extracted with ether.
The combined extracts are washed with water, dehydrated over sodium sulfate
and concentrated by evaporation under vacuum, and the product obtained is
isolated by distillation at 150°-155°C/0.8 mm Hg. 19.1 g (yield: 69%) of the
3-(4-fluorophenyl)-3-(pyrid-2-yl) propylamine are obtained.
1.15 g (5 mmol) of 3-(4-fluorophenyl)-3-(pyrid-2-yl)-propylamine and 1.59 g
(5 mmol) of N-benzoyl-diphenylimidocarbonate are stirred together in 20 ml
of methylene chloride for 15 min at room temperature. The solvent is distilled
off under vacuum and the residue is taken up with 30 ml of pyridine and then
heated under reflux for 60 min after the addition of 0.69 g (5.5 mmol) of 3-
(imidazol-4-yl)-propylamine. The reaction mixture is concentrated by
evaporation under vacuum and the residue is dissolved in dilute acid and
extracted with ether to remove the phenol formed in the reaction. Alkalization
of the aqueous phase with ammonia is followed by extraction with methylene
chloride, and the organic phase is washed with water, dehydrated over sodium
sulfate and concentrated by evaporation under vacuum. The crude product is
purified by preparative layer chromatography (silica gel 60 PF254, containing
gypsum, solvent: chloroform/methanol 99+1, ammoniacal atmosphere). 1.4 g
(yield 58%) of N-benzoyl-N'-[3-(4-fluorophenyl)-3-(pyrid-2-yl)propyl]-N"-[3-
(imidazol-4-yl)propyl]-guanidine a non-crystalline solid (foam) are obtained
(crystallisation from ethyl acetate).
0.97 g (2 mmol) of N-benzoyl-N'-[3-(4-fluorophenyl)-3-(pyrid-2-yl)propyl]-N"-
[3-(imidazol-4-yl)propyl]-guanidine are heated under reflux in 45 ml of 18%
hydrochloric acid for 6 h. When the reaction mixture has cooled down, the
benzoic acid formed is removed by extraction with ether, the aqueous phase is
evaporated to dryness under vacuum and the residue is dehydrated in a high
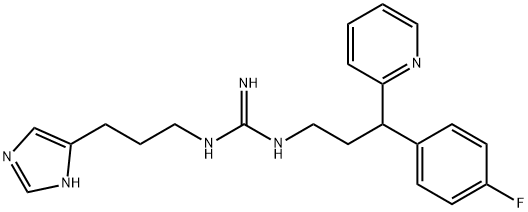
vacuum: 0.9 g (yield 92%) of a hygroscopic, non-crystalline N-[3-(4-fluorophenyl)-3-(pyrid-2-yl)propyl]-N'-[3-(imidazol-4-yl)propyl]-guanidine is
obtained.