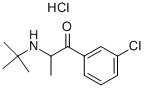
Description
Bupropion HCl (31677-94-7) is a clinically useful antidepressant that inhibits monoamine transporters DAT, NET, and SERT (Kis for dopamine uptake = 2.8, 1.4, 45 μM).1 Also acts as a noncompetitive antagonist at nicotinic acetylcholine receptors (AChRs).2 At higher concentrations (50 μM), stimulates pro-inflammatory cytokines and TLR2/TLR4 and JAK2/STAT3 in human peripheral blood mononuclear cells (PBMCs).3
Originator
Bupropion
hydrochloride,AroKor Holdings
Inc.
Uses
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Uses
Bupropion HC is used as an inhibitor of dopamine and SLC6A2. Bupropion is efficacious as an antidepressant because of its effects on nicotinic receptors, it is also used to promote smoking cessation.
Definition
ChEBI: Bupropion hydrochloride is an aromatic ketone.
Manufacturing Process
To ethyl magnesium bromide (2 L, 3 M) was added over 45 min with stirring
and cooling m-chlorobenzonitrile (688.0 g, 5 mole) in ether (2.5 L). The
resultant solution was heated under gentle reflux for 5 h. The reaction mixture
was hydrolyzed with cold dilute hydrochloric acid, the ether was distilled off,
and the aqueous solution was heated at 90°C for 1 h. The flask was then
cooled. The solid ketone that separated was washed with cold water and
recrystallized from methanol. The recrystallized m-chloropropiophenone,
melting point 39°-40°C, weighed 750.0 g.
In methylene chloride (3 L) was dissolved m-chloropropiophenone (698.0 g;
4.15 mole). The solution was stirred with charcoal (Darco) and magnesium
sulfate for 2 h and filtered. To it was added with stirring (662.0 g) of bromine
in methylene chloride (1 L). When the bromine color had faded completely,
the solvent was evaporated in vacuum and m-chloro-α-bromopropiophenone
was obtained as oil.
The m-chloro-α-bromopropiophenone was dissolved in acetonitrile (1300 ml).
To this, t-butylamine (733.0 g) in acetonitrile (1300 ml) was added while
keeping the temperature below 32°C. The reaction mixture was allowed to
stand over night. It was then partitioned between water (4200 ml) and ether
(2700 ml). The aqueous layer was extracted with a further portion of ether
(1300 ml). The combined ethereal layers were then washed with water (4200
ml) to which hydrochloric acid was added until the pH of the aqueous layer
was 9. The aqueous layer was separated and washed with ether (500 ml) and
then discarded. The combined ethereal layers were then stirred with ice
(560.0 g) and concentrated hydrochloric acid (324 ml). The ethereal layer was
separated and again washed with water (200 ml) and concentrated
hydrochloric acid (50 ml). These last two acid layers were combined and
concentrated in vacuum until crystals appeared. The solution was then chilled
to 5°C and filtered. The product was sucked dry, washed with acetone and
recrystallized from a mixture of isopropanol (3 L) and absolute ethanol (800
ml). The DL-m-chloro-α-t-butylaminopropiophenone hydrochloride so was
obtained, melting point 233°-234°C.
The DL-m-chloro-α-t-butylaminopropiophenone was obtained by treatment of
DL-m-chloro-α-t-butylaminopropiophenone hydrochloride with sodium
hydroxide.
brand name
Wellbutrin (GlaxoSmithKline); Zyban
(GlaxoSmithKline).
Therapeutic Function
Antidepressant; Smoking cessation aid
General Description
A certified solution standard suitable for use as starting material in calibrators or controls for a variety of LC/MS or GC/MS applications from forensic analysis and clinical toxicology to urine drug testing and pharmaceutical research. Bupropion is an antidepressant and smoking cessation drug sold under the trade names Wellbutrin
? and Zyban
?.
References
Urra et al. (2014), Presence and function of dopamine transporter (DAT) in stallion sperm: dopamine modulates sperm motility and acrosomal integrity; PLoS One, 9 e112834
Fryer and Lukas (1999), Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine; J. Pharmacol. Exp. Ther., 288 88
Karimollah et al. (2021), Revisiting bupropion anti-inflammatory action: involvement of the TLR2/TLR4 and JAK2/STAT3; Inflammopharmacology, 29 1101