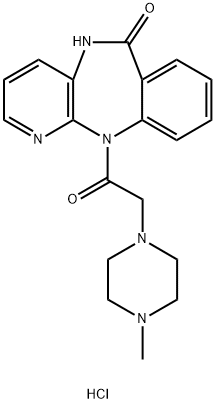
Description
Pirenzepine is an antagonist of M
1 muscarinic acetylcholine receptors (K
i = 11.48 nM). It is selective for M
1 over M
2, M
3, and M
4 receptors (K
is = 602.56, 151.36, and 199.53 nM, respectively). Pirenzepine inhibits ascending reflex contraction of the circular smooth muscle in isolated guinea pig ileal segments induced by intraluminal balloon inflation (IC
50 = 501.19 nM). It inhibits methacholine-induced increases in ileal pressure in guinea pigs (ID
50 = 724.44 nmol/kg). Pirenzepine inhibits oxotremorine-induced gastric ulcer, gastric acid secretion, and salivation in rats (ED
50s = 13, 37.5, and 620 μg/kg i.v., respectively). It prevents form-deprivation myopia (FDM) in a chick model of experimental myopia.
Chemical Properties
Solid
Originator
Microsules Bernabo, Microsules Bernabo
Uses
An antiulcerative. Tricyclic gastric-acid inhibitor.
Uses
Antiulcerative;M1 antagonist
Manufacturing Process
48.4 g of 5,11-dihydro-6H-pyrido[2,3-b][1,4]benzo-diazepin-6-one were refluxed in 900 ml of absolute dioxane for 15 minutes. Thereafter, over a period of 45 minutes, 28 ml of chloroacetyl chloride and 52 ml of triethylamine were simultaneously added dropwise to the mixture. The mixture was refluxed for eight hours and then vacuum-filtered after having cooled. The filtrate was evaporated in vacuum. The crystalline residue was recrystallized from acetonitrile in the presence of activated charcoal. MP: 212°-213°C (with decomposition). Yield: 85% of theory.
A mixture of 67.5 g of 11-chloroacetyl-5,11-dihydro-6H-pyrido[2,3b][1,4]benzodiazepin-6-one, 183 ml of N-methylpiperazine and 1.37 liters of absolute benzene was refluxed for 18 hours. Thereafter, the crystalline precipitate was vacuum filtered off, dissolved in aqueous 20% hydrochloric acid, the solution was evaporated in vacuum, the crystalline residue wasdissolved in 250 ml of water while heating, the solution was admixed with 150 ml of isopropanol and active charcoal, filtered, and 2.5 liters of isopropanol were added to the filtrate. After cooling, the precipitate was vacuum filtered off, yielding 70% of theory of the 5,11-dihydro-11-[(4'-methyl-1'-piperazinyl)acetyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one dihydrochloride, M.P. 257259°C (decomp.).
The free base of pirenzepine, obtained from the dihydrochloride by making an aqueous solution thereof alkaline with dilute sodium hydroxide and extracting it with chloroform, had MP: 226°-228°C after recrystallization from methanol/ether.
Therapeutic Function
Antiulcer, Antiemetic
General Description
Pirenzepine dihydrochloride is an anticholinergic agent. It is also considered as an antiulcer drug. It is used to treat myopia, gastric and duodenal ulcers. Pirenzepine dihydrochloride induces the dimerization of muscarinic M1 receptors.
Biochem/physiol Actions
Selective M1 muscarinic acetylcholine receptor antagonist.