Manufacturing Process
In a flask were placed 1,2-dichloroethane, AlCl 3 and acetyl chloride. The
reaction mixture is cooled to 10°C. Then biphenyl are loaded in little charge
during 3 h, avoiding to arise temperature over 20°C. The mixture is slowly
heated to 50°C and kept at this temperature for 4 h.
The obtained solution is then poured in a mixture of ice and of HCl 37%,
under stirring. Thereafter the mixture is let to stay for 12 h and phases
decantation occurs. Aqueous phase is extracted with dichloroethane. Organic
phases collected together are washed with H 2 O. Organic phase is dried on
CaCl 2 and then evaporated under vacuum till dryness. Solid residue is warm
dissolved in acetone, then is cooled and kept under stirring 1 night. Thereafter
it is let to stay 2 h in refrigerator and then the mixture is filtered, the solid is
washed with acetone and then with petroleum ether. The product is dried
under vacuum at 40°C to obtained the 4-phenyl-acetophenone.
The 4-phenyl-acetophenone was placed in a flask together with morpholine
and sulfur and the mixture heated under reflux for 5 h and a dark red solution
was obtained. After cooling to 50°C and addition of CH 3 OH a solid
precipitated. The mixture is kept under stirring at 50°C for 1 h, and then let
to stay 1 night in a refrigerator. Solid product is filtered and washed with
CH 3 OH and then dried under vacuum at 50°C to give the 4,4'-biphenylyl-
aceto-thiomorpholinamide.
The 4,4'-biphenylyl-aceto-thiomorpholinamide was placed together with H 2 O,
CH 3 COOH and HCl 37% in a flask. The mixture is heated under reflux for 8 h
and then poured in H 2 O. The mixture is cooled to 10°C and then filtered, the
solid product is pressed and washed with H 2 O. The solid product is dispersed
in H 2 O and NaOH 30% is added to the solution till pH value arises to 9.
Sulfur is then separated through filtration and the filtered solution is heated to
60°C, decolorized with active carbon, acidified to pH 2 with HCl 10%. The
solid product then separated is filtered, washed with H 2 O till pH neutral. The
product is then dried under vacuum at 60°C to give the 4,4'-biphenylyl-acetic
acid.
In a flask there were placed anhydrous ethanol, concentrated H 2 SO 4 and the
4,4'-biphenylyl-acetic acid. The mixture is heated under reflux for 3 h. The
reaction mixture is then poured in a solution of NaHCO 3 in of frozen water,
under stirring. Ester derivative precipitates and is filtered after 3 h stirring.
Solid product is washed with H 2 O till washing liquid is neutral. Drying is
carried out at 35°C under vacuum to give the ethyl 4,4'-biphenyl-acetate.
In a flask there are charged sodium hydride titre 80% and benzene. Keeping
temperature 20-30°C a solution of ethyl 4,4'-biphenylacetate and of crotyl
bromide in dimethylformamide is dropped during 30 min in the flask. Then the
reaction mixture is heated under reflux for 2 h, and subsequently cooled to
10°C. Thereafter H 2 O are charged and mixture stirred 15 min. Two phases
separate. Organic phase is washed with H 2 O and dried. The dried solution is
concentrated under vacuum till an oily residue consisting of ethyl ester of
diphenesenic acid.
The ethyl ester of diphenesenic acid residue is dissolved in ethanol. H 2 O and
KOH are then added and the mixture is heated under reflux for 2 h. The
alcohol is evaporated under vacuum. The residue is dissolved in H 2 O. Aqueous
solution is washed with petroleum ether. The solution is then decolorized with
active carbon and acidified slowly with HCl 10% till pH 2.1. After stirring at
room temperature for 4 h the solid product is filtered, washed with H 2 O till
washing liquid is neutral. The solution is then dried under vacuum at 50°C. So
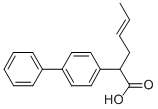
the 2-[4-biphenylyl]-4-hexenoic acid (diphenesenic acid), melting point 118-
119°C (crystallized from cyclohexane), boiling point 182-183°C is obtained.