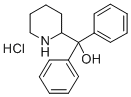
Description
Pipradrol is a mild stimulant containing a piperidine ring. Once used as a treatment for obesity and dementia, pipradrol is now regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
Manufacturing Process
A mixture of 48 g (0.167 mole) of α,α-diphenyl-2-pyridinemethanol hydrochloride (Emraert et al., Ber. 72B, 1188 (1939); 74B, 714 (1940), 160 ml of ethanol, and 3 0.5 g of Adams' platinum catalyst was shaken under an initial hydrogen pressure of 60 pounds. The theoretical amount of hydrogen was absorbed in 5 hours. The reaction mixture was refluxed, diluted with enough water to dissolve all the white solid, and filtered hot from the catalyst. The filtrate was cooled and filtered; yield of 38 g of α,α-diphenyl-alpha-(2piperidyl)methanol white product melting at 308-309°C with decomposition.
A mixture of 3.5 grams (0.013 mole) of the above α,α-diphenyl-alpha-(2piperidyl)methanol, 4 g (0.05 mole) of formaldehyde (37%), and 6 grams (0.1 mole) of formic acid was refluxed for 2 days. The reaction mixture was treated with 1.3 g (0.013 mole) of conc. hydrochloric acid and vacuum distilled on the steam bath. The residue was recrystallized from butanone to give the α,αdiphenyl-alpha-(2-piperidyl)methanol hydrochloride which melted at 228229°C (dec.).
Safety Profile
Poison by ingestion,
subcutaneous, intravenous, and
intraperitoneal routes. When heated to
decomposition it emits very toxic fumes of
HCl and NOx.