Manufacturing Process
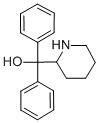
A mixture of 48 g (0.167 mole) of α,α-diphenyl-2-pyridinemethanol hydrochloride (Emraert et al., Ber. 72B, 1188 (1939); 74B, 714 (1940), 160 ml of ethanol, and 3 0.5 g of Adams' platinum catalyst was shaken under an initial hydrogen pressure of 60 pounds. The theoretical amount of hydrogen was absorbed in 5 hours. The reaction mixture was refluxed, diluted with enough water to dissolve all the white solid, and filtered hot from the catalyst. The filtrate was cooled and filtered; yield of 38 g of α,α-diphenyl-alpha-(2piperidyl)methanol white product melting at 308-309°C with decomposition.
A mixture of 3.5 grams (0.013 mole) of the above α,α-diphenyl-alpha-(2piperidyl)methanol, 4 g (0.05 mole) of formaldehyde (37%), and 6 grams (0.1 mole) of formic acid was refluxed for 2 days. The reaction mixture was treated with 1.3 g (0.013 mole) of conc. hydrochloric acid and vacuum distilled on the steam bath. The residue was recrystallized from butanone to give the α,αdiphenyl-alpha-(2-piperidyl)methanol hydrochloride which melted at 228229°C (dec.).
World Health Organization (WHO)
Pipradrol, a central nervous system stimulant, was introduced in
1955 for use as an anorexic agent. Pipradrol is controlled under Schedule IV of the
1971 Convention on Psychotropic Substances.
(Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV),
, , 1971)