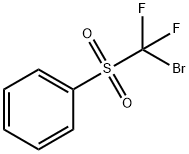
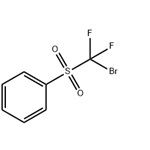
Description
The nucleophilic reactions of bromodifluoromethyl phenyl sulfone with electrophiles such as
aldehydes in the presence of TDAE affords (phenylsulfonyl)difluoromethyl-containing
synthetically useful intermediates. Palladium-mediated reactions of styrene derivatives, vinyl
ethers, and heteroaromatics with bromodifluoromethyl phenyl sulfone in the presence of
potassium carbonate affords the (phenylsulfonyl)difluoromethylated products.
Uses
Bromodifluoromethyl phenyl sulfone is an intermediate component in the synthesis of (phenylsulfonyl)difluoromethyl and can be used to prepare (phenylsulfonyl)difluoromethylated products by palladium-mediated reactions.
Uses
Bromodifluoromethyl Phenyl Sulfone can be used to prepare difluoromethyl ethers via difluoromethylation of alcohols.
Reactions
(1) Difluoromethylation of aldehydes.
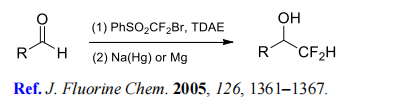
(2) Difluoromethylation of styrenes, vinyl ethers, and heteroaromatics.

References
[1] G.K. SURYA PRAKASH . Nucleophilic difluoromethylation and difluoromethylenation using bromodifluoromethyl phenyl sulfone[J]. Journal of Fluorine Chemistry, 2005. DOI:
10.1016/j.jfluchem.2005.07.011.[2] NAKIN SURAPANICH. ChemInform Abstract: Palladium-Mediated Heck-Type Reactions of [(Bromodifluoromethyl)sulfonyl]benzene: Synthesis of α-Alkenyl- and α-Heteroaryl-Substituted α,α-Difluoromethyl Phenyl Sulfones.[J]. ChemInform, 2013. DOI:
10.1002/chin.201314090.