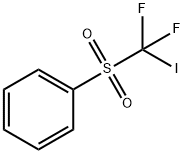
Description
A visible light-catalysed direct and regioselective (phenylsulfonyl) difluoromethylation of imidazo[1,2-A]pyridines and benzo[d]-imidazo[2,1-b]thiazoles was carried out by DifluoroiodoMethyl phenyl sulfone under mild conditions. This synthetic methodology enables the introduction of a CF2SO2Ph group in an efficient and regioselective reaction through C-H bond functionalization with a broad substrate scope in good to excellent yields.
Uses
Iododifluoromethyl
phenyl sulfone can be used for the difluoromethylation of alkenes and alkynes initiated by
triethylborane/air or arenediazonium salt/titanium chloride in moderate to good yields.
Reactions
(1) Difluoromethylation of terminal alkenes and alkynes.
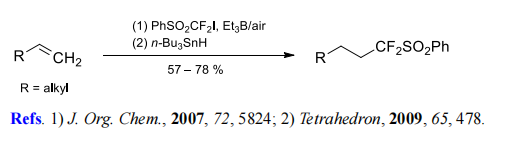
References
[1] GUO-JIE YIN; W. F; Mei Zhu. Visible-light mediated regioselective (phenylsulfonyl)difluoromethylation of fused imidazoles with iododifluoromethyl phenyl sulfone[J]. Heterocyclic Communications, 2017. DOI:
10.1515/hc-2017-0101.[2] YA LI. Radical (Phenylsulfonyl)difluoromethylation with Iododifluoromethyl Phenyl Sulfone[J]. The Journal of Organic Chemistry, 2007. DOI:
10.1021/jo070618s.