Description
A much milder difluoro(phenylsulfonyl)methylation reagent than difluoromethyl phenyl sulfone.
Under the action of Lewis bases such as such as tetrabutylammonium triphenyldifluorosilicate
(TBAT), potassium fluoride, potassium hydrodifluoride, and potassium carbonate,
difluoro(phenylsulfonyl)methyl can be transferred to aldehydes, ketones, alkyl halides, and
non-activated imines.
Reactions
(1) Difluoromethylation of aldehydes and ketones.
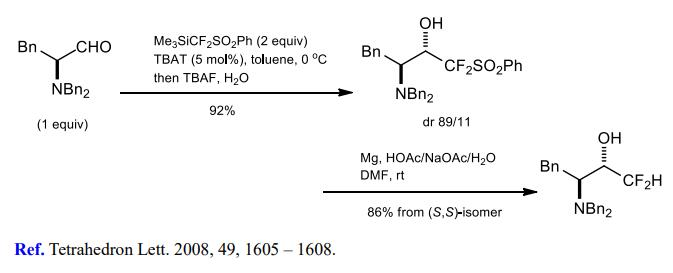
(2) Difluoromethylation of alkyl halides.
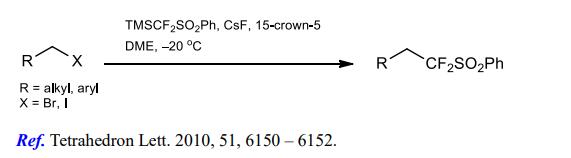
(3) Difluoromethylation of imines and enamines.
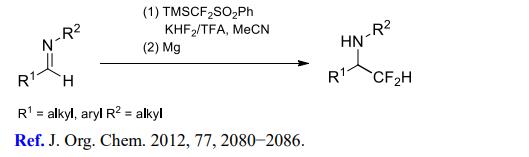
References
[1] H. TOMIOKA. Selective oxidation of a primary hydroxyl in the presence of secondary one[J]. Tetrahedron Letters, 1981, 49 1: 1605-1608. DOI:
10.1016/S0040-4039(01)90389-2[2] LINGUI ZHU. Nucleophilic (phenylsulfonyl)difluoromethylation of alkyl halides using PhSO2CF2SiMe3: preparation of gem-difluoroalkenes and trifluoromethyl compounds[J]. Tetrahedron Letters, 2010, 51 47: Pages 6150-6152. DOI:
10.1016/j.tetlet.2010.09.068.[3] MIKHAIL D. KOSOBOKOV. Reactions of Sulfur- and Phosphorus-Substituted Fluoroalkylating Silicon Reagents with Imines and Enamines under Acidic Conditions[J]. The Journal of Organic Chemistry, 2012, 77 4: 2080-2086. DOI:
10.1021/jo202669w.
![[difluoro(phenylsulfonyl)Methyl]triMethyl-Silane Structure](https://www.chemicalbook.com/CAS/GIF/536975-50-5.gif)


