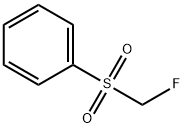
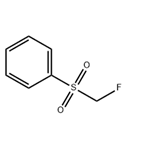
Description
Fluoromethyl phenyl sulfone is a useful nucleophilic monofluoromethylation reagent for the
synthesis of fluoromethyl alcohols and amines. In the nucleophilic reaction step, strong bases such
as LiHMDS and n-BuLi are used to generate the nucleophilic (phenylsulfonyl)fluoromethyl anion.
In the desulfonylation step, sodium/mercury amalgam and magnesium are the commonly used
reductive reagents. Besides, the addition reaction between fluoromethyl phenyl sulfone and
carbonyls can be used to prepare monofluoroaklenes via acylation–elimination.
Uses
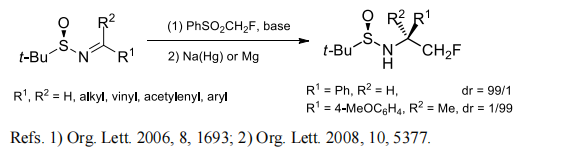
Fluoromethyl phenyl sulfone can be used to prepare monofluoroaklenes and chiral α-Monofluoromethyl Amines. Highly stereoselective nucleophilic monofluoromethylation of (R)-(tert-butanesulfinyl)imines with fluoromethyl phenyl sulfone was achieved to afford α-monofluoromethylamines with a nonchelation-controlled stereoselectivity mode. By using the same chemistry, (R)-(tert-butanesulfinyl)imines bearing a terminal tosylate (OTs) group can be converted to α-monofluoromethylated cyclic secondary amines with high stereoselectivity.
Reactions
(1) Monofluoromethylation of aldehydes and ketones.
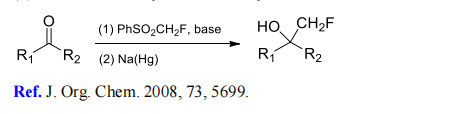
(2) Monofluoromethylation of aldimines and ketimines.
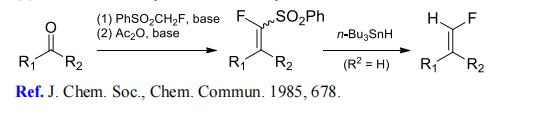
(3) Monofluoromethylenation of aldehydes and ketones.
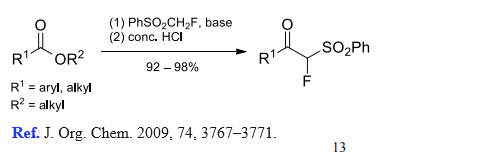
(4) (Phenylsulfonyl)fluoromethylation of esters.
References
[1] YA LI. Stereoselective Nucleophilic Monofluoromethylation of N-(tert-Butanesulfinyl)imines with Fluoromethyl Phenyl Sulfone[J]. Organic Letters, 2006. DOI:
10.1021/ol060322t.[2] M. INBASEKARAN. ChemInform Abstract: A NOVEL AND EFFICIENT SYNTHESIS OF FLUOROMETHYL PHENYL SULFONE AND ITS USE AS A FLUOROMETHYL WITTIG EQUIVALENT[J]. ChemInform, 1985. DOI:
10.1002/chin.198536160.[3] GOUVERNEUR V, LOZANO ó. Preparation of Chiral α-Monofluoromethyl Amines Using Fluoromethyl Phenyl Sulfone[C]. 1900. DOI:
10.1055/sos-SD-203-00578.