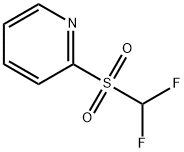
Description
Difluoromethyl 2-pyridyl sulfone, also known as Hu reagent, is a novel and efficient gem-difluoroolefination reagent for preparing gem-difluoroalkenes from both aldehydes and ketones. The fluorinated sulfinate intermediates during the gem-difluoroolefination is relatively stable, and can be halogenated in situ to afford bromo- and iododifluoromethyl compounds. It can also act as nucleophilic difluoro(sulfonato)methylation reagent for the synthesis of α,α-difluorosulfonates from aldehydes, and alkyl halides and triflates.
Chemical Properties
Melting point 48-50°C, boiling point 324.4+42°C, relative density 1.423+0.06 g/cm
3 , slightly soluble in water, casily soluble in acctonitrile and methanol.
Uses
Reagent is used in the olefination of ketones and aldehydes to form gem-difluoro olefins under basic conditions. Product is also used as a crucial intermediate toward making 1,1-difluorinated alkyl chains for the alkylation of heterocycles.
Uses
2-[(Difluoromethyl)sulfonyl]pyridine is a new novel gem-difluoroolefination reagent for both aldehydes and ketones.
Application
- Difluoromethyl 2-pyridyl sulfone can be used for the introduction of difluoromethylene, as a difluoroalkylation reagent to reacwith aldehydes and ketones to prepare difluoroalkenesIt can be used for the introduction of difluoromethyl, reacting with aryl zine and aryl iodine to preparedifluoromethyl aryl compounds, and conducting free radical addition reactions with olefins.
- It can be used as a nucleophilic difluoro (sulfonation) methylation reagent for synthesis from aldehydes.ketones, alkyl halides, trifluoromethanesulfonic acid esters, etc a,a- Difluoroalkyl sulfinates andsulfonates.
- It can also be used together with electrophilic iodination reagents and bromination reagents for theintroduction ofmonoiododifluoromethyl and monobromodifluoromethyl groups.
- It can also be used as a 2-pyridylation reagent.
Reactions
(1) gem-Difluoroolefination of aldehydes and ketones.
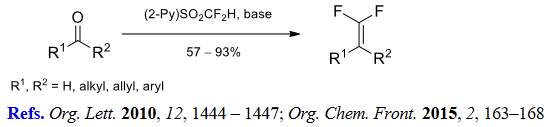
(2) Halodifluoromethylation of aldehydes and ketones.
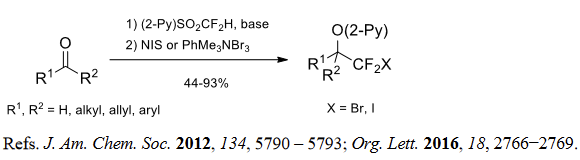
(3) (Fluorosulfonyl)difluoromethylation of aldehydes and ketones.
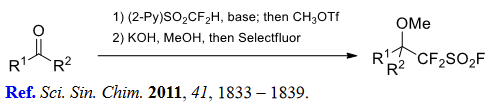
(4) Difluoro(sulfonato)methylation of alkyl halides and triflates.
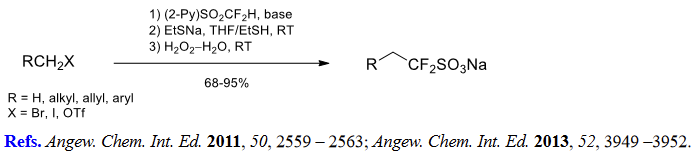
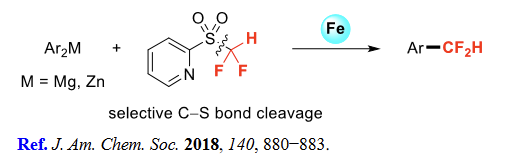
(5) Aromatic difluoromethylation
General Description
Difluoromethyl 2-pyridyl sulfone (2-PySO
2CF
2H) is a reagent used in the
gem-difluoroolefination of aldehydes and ketones. It is also used as a reagent in the nucleophilic difluoro(sulfonato)methylation of alcohols,
N-sulfinyl imines, and halides.
References
[1] WENJUN MIAO. Nickel-Catalyzed Reductive 2-Pyridination of Aryl Iodides with Difluoromethyl 2-Pyridyl Sulfone[J]. Organic Letters, 2021, 23 3: 711-715. DOI:
10.1021/acs.orglett.0c03939.
[2] WENJUN MIAO. Nucleophilic Iododifluoromethylation of Carbonyl Compounds Using Difluoromethyl 2-Pyridyl Sulfone[J]. Organic Letters, 2016, 18 11: 2766-2769. DOI:
10.1021/acs.orglett.6b01258.[3] YANCHUAN ZHAO. Difluoromethyl 2-Pyridyl Sulfone: A New gem-Difluoroolefination Reagent for Aldehydes and Ketones[J]. Organic Letters, 2010, 12 7: 1444-1447. DOI:
10.1021/ol100090r.