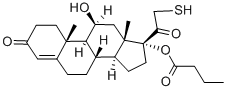
Manufacturing Process
3,20-Dione-11β-hydroxypregn-4-ene-21-thioacetate-17-butyrate (or
hydrocortisone): 10.0 g (0.129 mole) of S-thioacetic acid and 220 ml of
hexametapol are introduced into a reactor 27.7 ml of a 4.65 N sodium
methylate methanolic solution (0.129 mole) are introduced, accompanied by
stirring, at a temperature close to 20°C and then the beige solution is stirred
for 1 hour at ambient temperature. Within 10 minutes, a solution of 44.0 g
(0.086 mole) of cortisol-21-mesylate-17-butyrate is introduced into 440 ml of
hexametapol. The solution is stirred for 2.5 hours at ambient temperature.
The orange solution is precipitated in 8 liters of ice water. The insoluble
substances formed are filtered and then taken up in methyl ether. The
ethereal solution is extracted twice with 250 ml of 1 N sodium hydroxide
solution and then three times with 500 ml of saturated sodium chloride
solution. After drying the ethereal phase, the solvent is eliminated by
distillation. The residue (39 g) is purified by column chromatography with the
aid of 1 kg of "Florisil''. Elution by a mixture of dichloromethane and acetone
95:5 (v/v) makes it possible to collect 21 g of purified product. This product is
finally recrystallized in 170 ml of a mixture of methanol and water 8:2 (v/v).
Weight=19 g. Yield=44.3%. Melting point: 130°C; the structure of prepared
compound is confirmed by NMR spectrum.