Definition
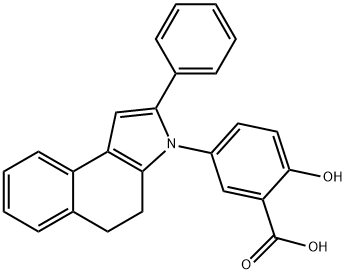
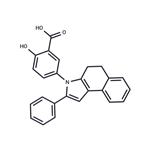
ChEBI: Fendosal is a benzoindole that is 4,5-dihydro-3H-benzo[e]indole in which the nitrogen is substituted by a 3-carboxy-4-hydroxyphenyl group. A non-narcotic analgesic and non-steroidal anti-inflammatory drug, it has greater analgesic and inflammatory responses than aspirin but with less gastrointestinal toxicity. It has a role as a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is a member of pyrroles, a benzoindole and a monohydroxybenzoic acid.
Manufacturing Process
To a stirred refluxing solution of 20.2 g (0.1 mole) of 1-(1-pyrrolidino)-3,4-
dihydronaphthalene and 50 ml of toluene was added dropwise during 30 minutes under nitrogen a solution of 20.1 g (0.1 mole) of phenacyl bromide in
65 ml of dry toluene. The mixture was heated under reflux for 6 hours, diluted
with 50 ml of water, refluxed for 4 hours, and cooled. The layers were
separated and the aqueous phase was extracted with benzene. The organic
solution was dried over sodium sulfate and concentrated to a semi-solid.
Trituration with cold 30°-60° petroleum ether gave 23.6 g (78%) of solid, 2-
phenacyl-1-tetralone, MP: 73°-76°C. Recrystallization from 60°-90° petroleum
ether raised the melting point to 87°-88°C.
A mixture of 20.0 g (0.076 mole) of 2-phenacyl-1-tetralone, 11.6 g (0.076
mole) of 5-aminosalicyclic acid, and 70 ml of glacial acetic acid was heated
under reflux for 4 hours, cooled, diluted with 10 ml of water and filtered. The
filter cake was washed with water and dried to provide 15.5 g of solid, 5-(4,5-
dihydro-2-phenyl-3H-benz[e]indol-3-yl)-2-hydroxy-benzoic acid. MP: 215°-
218°C. Recristallization from benzene-cyclohexane gave 6.5 g (22%) of yellow
crystals, MP: 245°-247°C.