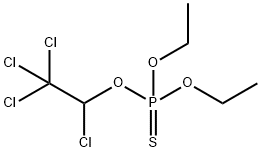
Chemical Properties
Gray to reddish-brown granular solid or colorless liquid. Mild sulfur-like odor.
Chemical Properties
Chlorethoxyfos is a colorless liquid. Technical chlorethoxyfos
is a light-to- dark brown liquid. Stable at room temperature for >18 months; stable at 55°C for 2 weeks. Chlorethoxyfos is characterized as having a “strong” odor.
Uses
Chlorethoxyfos is a soil-applied organophosphorus insecticide
used to control various soil insects (e.g. corn rootworms) in maize.
Uses
Chlorethoxyfos is an organophosphorous pesticide/insecticide.
Definition
ChEBI: Chlorethoxyfos is an organic thiophosphate, an organothiophosphate insecticide and an organochlorine insecticide. It has a role as an agrochemical and an EC 3.1.1.7 (acetylcholinesterase) inhibitor.
Agricultural Uses
Insecticide: Chlorethoxyfos is a restricted use organophosphate insecticide registered for use in the U.S. on field corn, seed corn, sweet corn, and popcorn for the control of corn rootworms, wireworms, cutworms, seed corn maggots,
white grubs, and symphylans. A U.S. EPA restricted Use Pesticide (RUP). Not listed for use in EU countries.
Trade name
DPX-43898®; FORTRESS 2.5G; FORTRESS® 5G; SD 208304®
Potential Exposure
An organophosphate insecticide,
chlorethoxyfos is a restricted use organophosphate insecticide registered for use in the United States on field corn,
seed corn, sweet corn, and popcorn for the control of corn
rootworms, wireworms, cutworms, seed corn maggots,
white grubs, and symphylans. A United States
Environmental Protection Agency Restricted Use Pesticide
(RUP). Not currently listed for use in EU countries.
Carcinogenicity
According to an EPA summary,
an 18 month study in which mice were given diets with
chlorethoxyphos (doses not provided) was negative for
carcinogenicity . A 2 year rat feeding (doses not
provided) study showed a slight, nonsignificant increase in
kidney tumors, and EPA concluded “the study was adequate
for carcinogenicity testing” but that it was difficult to clearly
interpret the data as showing either the presence or the
absence of a carcinogenic effect .
Metabolic pathway
Chlorethoxyfos undergoes extensive degradation and metabolism in
water, soil, plants and animals. Cleavage of the P-O-tetrachloroethyl bond
is the major degradation reaction of chlorethoxyfos. Dechlorination and
oxidation of the tetrachloroethyl moiety yielded chloral, dichloroacetic
acid and trichloroacetic acid as major degradation products. The extensive
metabolism of chlorethoxyfos and its degradation products yielded
14CO2 and amino acids (glycine, serine, etc.). Oxidative desulfuration to
yield chlorethoxyfos-oxon or O-de-ethylation reactions were not observed.
Shipping
UN2783 Organophosphorus pesticides, solid,
toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
UN3018 Organophosphorus pesticides, liquid, toxic,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Degradation
Chlorethoxyfos is susceptible to alkaline hydrolysis. The DT
50 values of
chlorethoxyfos in buffers at pH 5, 7 and 9 at 25 °C were 72, 59 and 4.3
days, respectively (Hawhs et al., 1988a). The primary hydrolytic
degradation reaction was the cleavage of the P-O-tetrachloroethyl bond.
The major [
14C-tetrachloroethyl]chlorethoxyfos degradation product observed
in pH 5 and 7 aqueous solutions was chloral (trichloroacetaldehyde,
2), formed probably via the tetrachloroethanol (3) intermediate. In
alkaline solution (pH 9), the principal product was dichloroacetic acid (4),
formed probably via the dehydrochlorinated intermediate (5).
Chlorethoxyfos degraded slowly in aqueous solution when exposed
to UV light at 25 °C [DT
50 ca. 27 days (continuous irradiation) vs. 19
days (dark control)] (Hawkins et al., 1988b). Cleavage of the P-O-
tetrachloroethoxy bond yielded chloral (2) as the primary aqueous
photolysis product.
Incompatibilities
May react violently with antimony(V)
pentafluoride. Incompatible with lead diacetate, magnesium, silver nitrate. In the presence of strong reducing
agents such as hydrides, organophosphates form highly
toxic and flammable phosphine gas. Contact with oxidizers
can cause the release of toxic oxides of phosphorus.
Waste Disposal
Destruction by alkali hydrolysis or incineration. May also be mixed with flammable solvent and sprayed into an incinerator equipped with after
burner and scrubber. Containers must be disposed of properly by following package label directions or by contacting
your local or federal environmental control agency, or by
contacting your regional EPA office. Afterburner