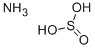
Ammonium bisulfite
- Product NameAmmonium bisulfite
- CAS10192-30-0
- CBNumberCB3298734
- MFH5NO3S
- MW99.11
- EINECS233-469-7
- MDL NumberMFCD00041958
- MOL File10192-30-0.mol
Chemical Properties
| Melting point | 147°C |
| Density | 2.03g/cm3 |
| storage temp. | Refrigerator, Under inert atmosphere |
| solubility | Water (Soluble) |
| form | colorless crystals |
| color | colorless crystals, crystalline |
| Water Solubility | soluble |
| Cosmetics Ingredients Functions | HAIR WAVING OR STRAIGHTENING PRESERVATIVE REDUCING |
| Cosmetic Ingredient Review (CIR) | Ammonium bisulfite (10192-30-0) |
| InChI | InChI=1S/H3N.H2O3S/c;1-4(2)3/h1H3;(H2,1,2,3) |
| InChIKey | ZETCGWYACBNPIH-UHFFFAOYSA-N |
| SMILES | S(=O)(O)O.N |
| LogP | -1.591 (est) |
| CAS DataBase Reference | 10192-30-0(CAS DataBase Reference) |
| EWG's Food Scores | 2-3 |
| FDA UNII | 8MW949D9GE |
| EPA Substance Registry System | Ammonium bisulfite (10192-30-0) |
Safety
| Symbol(GHS) |

|
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P |
| RIDADR | UN 2693 |
| TSCA | TSCA listed |
| HazardClass | 8 |
| PackingGroup | III |
| Hazardous Substances Data | 10192-30-0(Hazardous Substances Data) |


