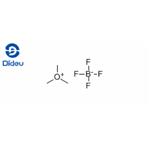
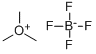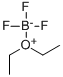Description
Trimethyloxonium tetrafluoroborate (TMO), a trialkyloxonium salt, is a white salt at ambient temperature that can be added directly to reaction media to carry out the methylation of reactive, acidic analytes. The main drawback associated with the employment of TMO is the generation of tetrafluoroboric acid (HBF4), which may interfere with the analysis of acid-sensitive analytes and cause column degradation over time. However, the effects of this acid can be countered by neutralization with inorganic bases like sodium or potassium bicarbonate during the sample preparation step before instrument injection. This approach has proven crucially useful in analysing phosphonic and sulfonic acids related to organophosphorus-based nerve agents. TMO could be employed as a derivatizing agent in the methylation of a panel of environmentally relevant chlorinated phenols. It is also used in neutral and basic solutions for the selective methylation of the carboxyl groups of protohemin IX, hematohemin, and heme a[1-2].
Chemical Properties
This substance is a white to off-white crystals that is prone to water sensitivity and displays a level of hygroscopicity.
Uses
Trimethyloxonium Tetrafluoroborate is a trialkylated oxonium used as a methylation agent in organic synthesis. It is a reagent for the methylation of hydroxyl groups recently used in a complex, multistep synthesis directed towards spirastrellolide, a marine natural product.
Uses
Trimethyloxonium tetrafluoroborate act as a avmethylating agent and activates C-X multiple bonds. It is involved in the esterification of polyfunctional carboxylic acids. It acts as a catalyst in the polymerization of cyclic sulfides and ethers. Further, it is used in Beckmann rearrangement of oximes.
Health Hazard
Trimethyloxonium Tetrafluoroborate is a potent alkylating agent, with its non-volatile nature reducing associated hazards. Nevertheless, it produces strong acids upon contact with water, so it is important to exercise caution and prevent water contact during its application.
Synthesis
The general procedure for the preparation of trimethoxonium tetrafluoroborate is as follows (adapted with minor modifications from TJ. Curphey, Org. Synth., Coll. vol. 6, p. 1019 (1988)):
1. add 80 mL of dichloromethane (DCM) to a 500 mL three-necked round-bottomed flask (equipped with an N2 inlet, 60 mL pressure-equalizing dropping funnel, magnetic stirring bar, and rubber septum, and marked at approximately 190 mL) that has been pre-dried in an oven, under the protection of dry nitrogen gas.
2. 33.3 mL (270 mmol) of boron trifluoride ether compound was added, followed by cooling the mixture in a dry ice-acetone bath.
3. condense the DME into the DCM solution through a needle inserted through the rubber septum, which should remain just below the liquid level until the total volume of liquid reaches the 190mL mark.
4. add 24.1mL (307mmol) of epichlorohydrin dropwise over approximately 15 minutes with vigorous stirring (note: the mixture will become very viscous and may need to be rotated manually to ensure adequate stirring).
5. Remove the cooling bath and continue to vigorously stir the mixture overnight at room temperature.
6. Upon completion of the reaction, the resulting solid product was collected by filtration through a medium glass-filled Brinell's funnel, which had been pre oven-dried, under a stream of N2 gas.
7. The flask and filter were rinsed with dichloromethane (2 x 100mL) to remove the solids.
8. After drying under nitrogen protection, 29.4 g (98% yield) of free flowing white solid trimethoxonium tetrafluoroborate was obtained.
9. Store the product in a pre-oven dried glass vial in a nitrogen protected refrigerator.
References
[1] Carlos A Valdez, Roald N Leif, Edmund P Salazar. “Trimethyloxonium-mediated methylation strategies for the rapid and simultaneous analysis of chlorinated phenols in various soils by electron impact gas chromatography-mass spectrometry.” Scientific Reports (2022): 1401.
[2] R T Dean, D E Hultquist, L J DeFilippi. “Esterification of hemins with trimethyloxonium tetrafluoroborate.” Analytical biochemistry 76 l (1976): 1–8.