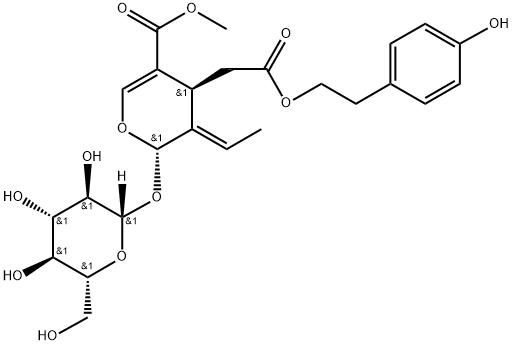
Definition
ChEBI: Ligstroside is a secoiridoid glycoside that is the methyl ester of 3,4-dihydro-2H-pyran-5-carboxylic acid which is substituted at positions 2, 3, and 4 by hydroxy, ethylidene, and carboxymethyl groups, respectively and in which the anomeric hydroxy group at position 2 has been converted into its beta-D-glucoside and the carboxylic acid moiety of the carboxymethyl substituent has been converted to the corresponding 4-hydroxyphenethyl ester (the 2S,3E,4S stereoisomer). An important phenolic compound present in olive cultivars. It has a role as a plant metabolite and an antineoplastic agent. It is a secoiridoid glycoside, a methyl ester, a diester, a member of pyrans, a member of phenols and a beta-D-glucoside.
Biological Activity
Ligstroside is a natural product derived from leaves of Olea europaea. Ligstroside displays several potentially beneficial anti-inflammatory properties by exhibiting selective inhibition effect on COX-1 enzyme of arachidonate cascade metabolism. Ligstroside generates a reduction of the PGE2 (prostaglandin E2) levels in activated mouse macrophage (IC50 = 48.53 μM) and also reduction of the TXB2 (thromboxane B2) levels in human platelets (IC50 = 122.63 μM). Moreover, Ligstroside exhibits inhibition activity of NO production in LPS-activated RAW264.7 macrophages (17.8% at 30 μM and 40.7% at 100 μM) without any cytotoxic effect in these cells., Additionally, Ligstroside exhibits in vitro antiviral activity against parainfluenza type 3 virus (IC50 = 15.6 μM).