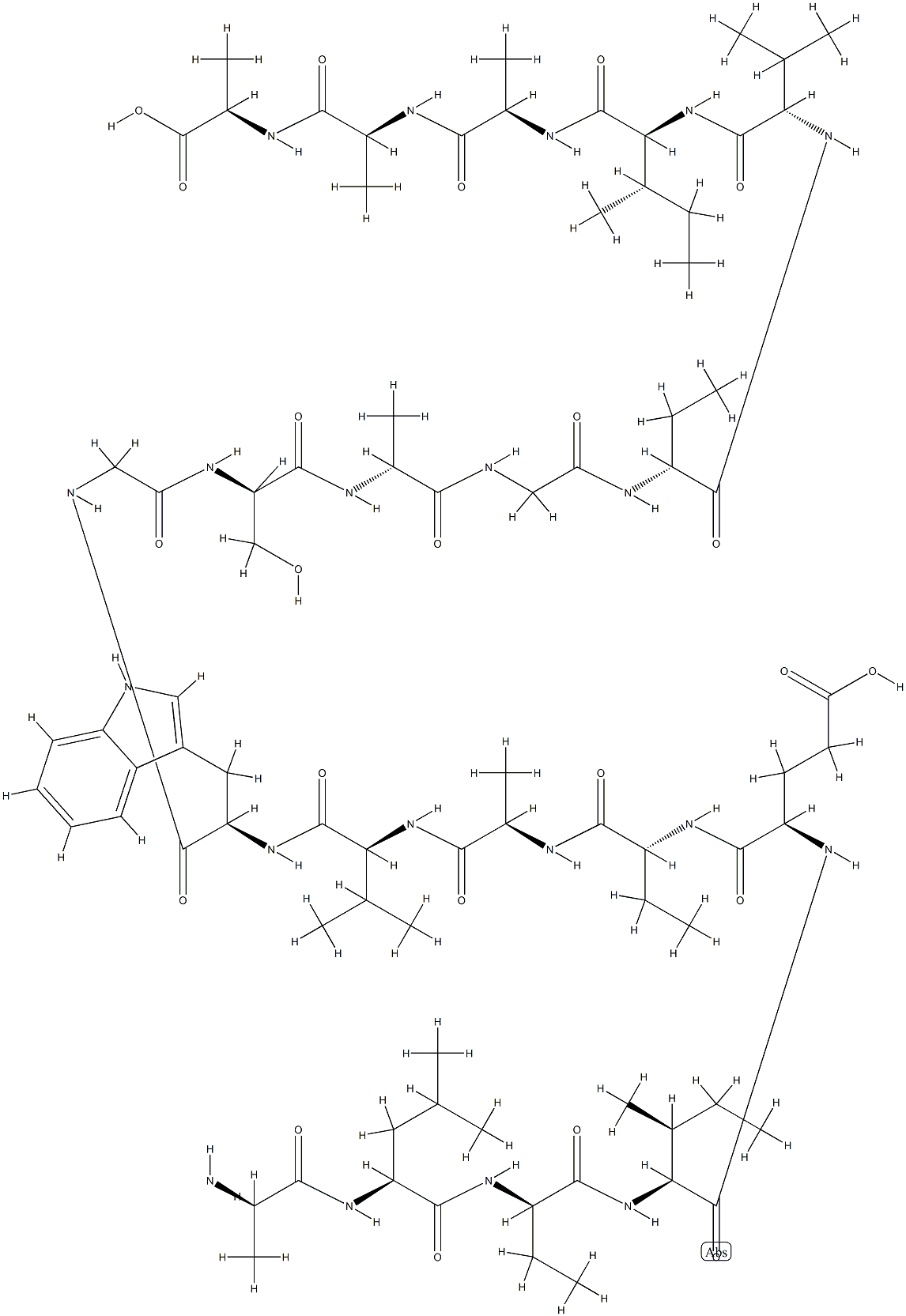
gardimycin
- Product Namegardimycin
- CAS59165-34-3
- CBNumberCB31370367
- MFC81H124N20O24S4
- MW1890.23
- MDL NumberMFCD01772577
- MOL File59165-34-3.mol
Chemical Properties
| Boiling point | 2194.7±65.0 °C(Predicted) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in ethanol;methanol;DMSO;dimethyl formamide |
| form | solid |
| pka | 2.74±0.70(Predicted) |
| EWG's Food Scores | 1 |
gardimycin Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| Cayman Chemical 19940 | 1mg | $335 | Actagardin ≥98% |
Buy |
| Cayman Chemical 19940 | 5mg | $1169 | Actagardin ≥98% |
Buy |
| TRC A191703 | 1mg | $245 | Actagardin |
Buy |
| TRC A191703 | 2.5mg | $495 | Actagardin |
Buy |
| American Custom Chemicals Corporation API0013314 | 5MG | $496.76 | GARDIMYCIN 95.00% |
Buy |
gardimycin Chemical Properties,Usage,Production
Originator
Actagardine,Lepetit S.p.A.Uses
Actagardin is a high molecular weight tetracyclic antibiotic produced by several species of Actinoplanes. Actagardin is a lantibiotic in which the macrocyclic rings are formed by thioether, rather than disulphide, bridges. Actagardin is a potent Gram positive and Gram negative antibiotic. Actagardin acts by inhibition of peptidoglycan biosynthesis.Manufacturing Process
The antibiotic actagardine is produced by aerobically pre-culturing of the strain Actinoplanes garbadinensis ATCC 31049 in a nutrient medium.A shake flask culture may have the following composition in g/L: meat extract 3.0; yeast extract 10.0; calcium carbonate 4.0; Starch 25.0; tap water q.s. to 1000 ml. The flasks are shaken for about 24 h at about 28°-30°C an then the pre-cultures 1 L are used to inoculate jar fermentors each containing 10 L of the following nutrient medium, g: meat extract 40.0; peptone 40.0; yeast extract 10.0; sodium chloride 25.0; soybean meal 100.0; glucose 500.0; calcium carbonate 50.0; tap water q.s. to 10 L.
The fermentation batches are incubated aerobically under stirring at 28°- 30°C. At intervals the antibiotic activity is assayed microbiologically by the agar diffusion method using Sarcina lutea as the test organism. The maximum activity is reached after 96-120 h of fermentation.
The fermentation broth is adjusted at pH 8.0 and then filtered using Hyflo super-cell as a filter aid. The mycelium is discarded and the filtrate is extracted with an amount of butanol corresponding to about 0.5 of its volume. The organic phase is separated from the aqueous one, and, after washing with acidic water (pH 4.0) is concentrated to about 1:10 of its original volume and allowed to stand for 10-12 h at a temperature of 3°-6°C. A crude precipitate forms, which is collected on filter, washed with butanol and dried under vacuum at room temperature: yield 3.0 g.
Chromatographic assays on Whatman paper N1 or on silica-gel of this crude precipitate, and subsequent microbiological development of the spots by using Staphylococcus aureus as the detecting system, indicate the presence of two components which are defined as metabolite A and metabolite B (gardimycin): they have different R values which depend on the nature of the employed eluting system. The crude mixture is further purified by dissolving in about 30 ml of water. The resulting solution is dialyzed for about 16 h against distilled water and then concentrated to small volume under vacuum. 1.5 g of rough antibiotic substance are obtained, which still is a mixture of metabolite A and gardimycin. The two antibiotic substances are separated and purified by several countercurrent extractions, by relying upon the different partition coefficients of component A and gardimycin in the predetermined solvent system. The employed solvent system consists of butanol:sodium-potassium phosphate buffer M/15 pH 7.2: hexane in the ratio 1:1:0.1; the partition coefficients in this medium of metabolite A and gardimycin are 0.3 and 0.8 respectively. After 100 extractions, 0.45 0 g of gardimycin as its monosodium salt, melting point 260°C (dec.) are obtained.
1.0 g of gardimycin monosodium salt is dissolved in 150 ml of water. The resulting solution is brought to pH 2.5 by adding aqueous 10% hydrochloric acid and is then extracted two times with 75 ml of butanol saturated with water. The butanol extracts are collected and concentrated in vacuum at 45°C to a volume corresponding to 1:20 of the initial volume. After standing at 4°C for 12 h a precipitate forms, which is collected, washed with light petroleum and dried in vacuo at 40°-45°C. 0.950 g of gardimycin (free acid), melting point 250°-300°C (dec.) are obtained.
Therapeutic Function
AntibioticBiological Activity
actagardin is a tetracyclic antibiotic.actagardin is a tetracyclic antibiotic produced by several species of actinoplanes.in vitro
during the repeated fermentations of actagardin, two new compounds, named d and e, were isolated. compound d was found to be twice as active as actagardin against streptococcus pyogenes c 203 and four times more active than actagardin against staphylococcus aureus atcc 6538, s. pneumoniae uc 41, and s. aureus tour in the in-vitro tests [1].in vivo
animal study showed that compound d was also more effective than the parent compound actagardin against experimental infections in mice with either s. pyogenes c 203 or s. aureus tour, after sc administration. however, compound d was ineffective at doses up to 150 mg/kg when given orally to mice infected with s. pyogenes c 203. moreover, compound d (ld50 1,250 mg/kg, mice, ip) was about 2.5 times more toxic than actagardine (3,310 mg/kg). in addition, compound e showed practically no in vitro activity up to 50 μg/ml against the tested organisms [1].References
[1] malabarba a, landi m, pallanza r, cavalleri b. physico-chemical and biological properties of actagardine and some acid hydrolysis products. j antibiot (tokyo). 1985 nov;38(11):1506-11.Preparation Products And Raw materials
gardimycin Suppliers
Global(16)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| 16314854226; +16314854226 |
inquiry@bocsci.com | United States | 19741 | 58 | |
| tp@aladdinsci.com | United States | 52924 | 58 | ||
| 021-65675885 18964387627 |
info@efebio.com | China | 9804 | 58 | |
| 86-(0)29-85992781 17792393971 |
info@lynnchem.com | China | 4587 | 58 | |
| 44-20819178-90 02081917890 |
info@novachemistry.com | United Kingdom | 4381 | 58 | |
| +86-400-002-6226 +86-13028896684; |
sales@rrkchem.com | China | 57423 | 58 | |
| 18818260767 | sales@chemegen.com | China | 11218 | 58 | |
| 400-820-6829 | sales@mitachieve.com | China | 8860 | 58 | |
| 021-69568360 18916172912 |
order@med-bio.cn | China | 8140 | 58 | |
| 021-58000709 15900491054 |
info@scrbio.com | China | 9502 | 58 |
59165-34-3, gardimycinRelated Search
- TRIS(2,2,6,6-TETRAMETHYL-3,5-HEPTANEDIONATO)EUROPIUM(III)
- TERT-BUTYL ISOCYANIDE
- Tris(2,4-pentanedionato)chroMiuM(III)
- Cupric acetylacetonate
- 2,4-PENTANEDIONE, SILVER DERIVATIVE
- Tosylmethyl isocyanide
- 1,1,3,3-TETRAMETHYLBUTYL ISOCYANIDE
- METHYL ISOCYANOACETATE
- Aluminum acetylacetonate
- N-BUTYLISOCYANIDE
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine