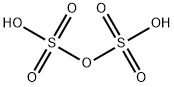
Description
Disulfuric acid has the molecular
formula of H2S2O7 where the S-atom is in the+5 oxidation
state. It is a major constituent of fuming sulfuric acid (also known as
“oleum”) described by the formula ySO3·H2O where y
is the total molar sulfur trioxide content. They can also be described by the formula: H2SO4·xSO3,
which are also minor constituents of liquid anhydrous
sulfuric acid due to the equilibria:
H2O+SO3→H2SO4+SO3→H2S2O7
Thus, the disulfuric acid is prepared by reacting
excess SO3 with sulfuric acid:
H2SO4+SO3→H2S2O7
Disulfuric acid is a strong acid and protonates
sulfuric acid in the (anhydrous) sulfuric acid solvent
system. An alternative IUPAC name is (sulfo-oxy)sulfonic
acid.