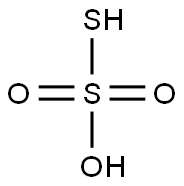
Physical properties
Thiosulfuric acid is a sulfur oxoacid. The free acid
cannot be prepared by acidifying thiosulfate salts
as the acid readily decomposes in water. Anhydrous methods of producing
the acid are:
H2S+ SO3→H2S2O3·nEt2O (in diethyl ether at 78°C)
Na2S2O3+2HCl/2NaCl+H2S2O3·2Et2O
(in diethyl ether at 78°C)
HSO3Cl+H2S→HCl+H2S2O3 (low temperature)
The anhydrous acid also decomposes below 0°C:
H2S2O3→H2S+ SO3
There is another isomeric form, a white crystalline
adduct of hydrogen sulfide and sulfur trioxide, H2S·SO3, which can also be prepared at low temperature.