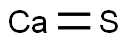
CALCIUM SULFIDE
- Product NameCALCIUM SULFIDE
- CAS20548-54-3
- CBNumberCB3391370
-
MFCaHS
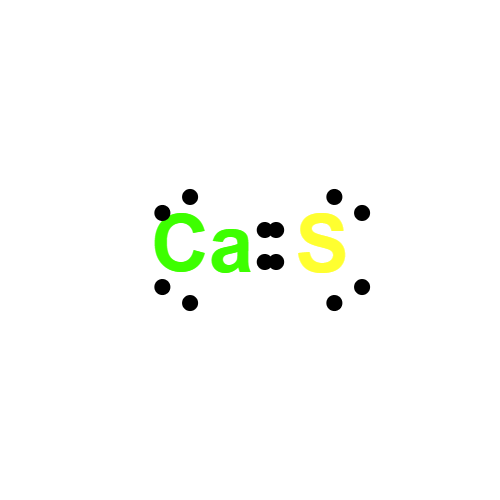
Lewis structure
- MW73.15
- EINECS243-873-5
- MDL NumberMFCD00015990
- MOL File20548-54-3.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 2000 °C | ||||||||||||||
| Density | 2.6 | ||||||||||||||
| refractive index | 2.12 | ||||||||||||||
| solubility | slightly soluble in H2O; insoluble in ethanol | ||||||||||||||
| form | Powder | ||||||||||||||
| Specific Gravity | 2.5 | ||||||||||||||
| color | White | ||||||||||||||
| Water Solubility | Slightly soluble in water. Insoluble in alcohol. | ||||||||||||||
| Sensitive | Moisture Sensitive | ||||||||||||||
| Crystal Structure | NaCl type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Merck | 14,1707 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Cosmetics Ingredients Functions | DEPILATORY | ||||||||||||||
| InChI | 1S/Ca.S | ||||||||||||||
| InChIKey | JGIATAMCQXIDNZ-UHFFFAOYSA-N | ||||||||||||||
| SMILES | S=[Ca] |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H228-H315-H319-H335-H400 | |||||||||
| Precautionary statements | P210-P273-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 31-36/37/38-50 | |||||||||
| Safety Statements | 28-61 | |||||||||
| RIDADR | UN 3179 4.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| TSCA | TSCA listed | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | III | |||||||||
| Storage Class | 4.1B - Flammable solid hazardous materials | |||||||||
| Hazard Classifications | Aquatic Acute 1 Eye Irrit. 2 Flam. Sol. 1 Skin Irrit. 2 STOT SE 3 | |||||||||
| NFPA 704: |
|