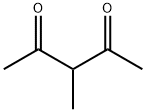
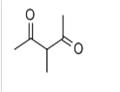
Chemical Properties
clear colourless to yellow liquid
Uses
3-Methyl-2,4-pentanedione involves in asymmetric substitution of 1,3-diphenyl-2-propenyl acetate catalyzed by amphiphilic resin-supported monodentate phosphine ligands can occur.
Synthesis Reference(s)
Chemistry Letters, 8, p. 45, 1979
Journal of the American Chemical Society, 90, p. 2421, 1968
DOI: 10.1021/ja01011a039Organic Syntheses, Coll. Vol. 5, p. 785, 1973
General Description
Peroxynitrite promoted aerobic oxidation of 3-methyl-2,4-pentanedione has been reported. The kinetics of the reaction of OH radicals with 3-methyl-2,4-pentanedione has been investigated in the gas-phase using a pulsed laser photolysis-laser induced fluorescence technique. 3-methyl-2,4-pentanedione participates in asymmetric substitution of 1,3-diphenyl-2-propenyl acetate catalyzed by amphiphilic resin-supported monodentate phosphine ligands.