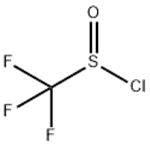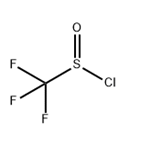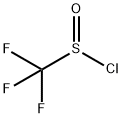
TRIFLUOROMETHYL SULFINYL CHLORIDE
- Product NameTRIFLUOROMETHYL SULFINYL CHLORIDE
- CAS20621-29-8
- CBNumberCB31177388
- MFCClF3OS
- MW152.52
- MDL NumberMFCD10566393
- MOL File20621-29-8.mol
Chemical Properties
| Boiling point | 149.4±35.0 °C(Predicted) |
| Density | 1.852±0.06 g/cm3(Predicted) |
| solubility | soluble in No data available |
TRIFLUOROMETHYL SULFINYL CHLORIDE Chemical Properties,Usage,Production
Chemical Properties
This product is liquid, bp30 ℃, volatile at room temperature, soluble in inert organic solvent, and decomposes in contact with water.Uses
Trifluoromethyl sulfinyl chloride is an intermediate for the insecticide fipronil.Synthesis
The preparation method of Trifluoromethyl sulfinyl chloride is that CF3Cl is reacted with sodium dithionite to generate sodium trifluoromethyl sulfite, and then reacted with thionyl chloride to generate a product. Reaction equation: CF3Cl1+Na2S2O4→CF3SO2Na[SOCl2]→CF3SOClIt can also be reacted with potassium trifluoroacetate and sulfur dioxide in a solvent to generate Potassium trifluoromethyl sulfoxide, and then react with thionyl chloride to produce the product. Reaction equation: CF3COOK+SO2→CF3SO2K→CF3< /SUB>SOCl
Preparation Products And Raw materials
Raw materials
TRIFLUOROMETHYL SULFINYL CHLORIDE Supplier
Global(93)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-371-66670886 | info@dakenam.com | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21632 | 55 | |
| +86-0571-85586753; +8613336034509 |
sales@fluoropharm.com | China | 1376 | 60 | |
| +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29871 | 58 | |
| 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 | |
| +86-21-52699951; +8613917686115 |
sales@beyondindustriesgroup.com | China | 699 | 58 | |
| +86-021-61551413 +8618813727289 |
contact@trustwe.com | China | 5738 | 58 | |
| +8613367258412 | ada@ipurechemical.com | China | 10319 | 58 | |
| +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
20621-29-8, TRIFLUOROMETHYL SULFINYL CHLORIDERelated Search
- Sodium trifluoromethanesulfinate
- Zinc TrifluoroMethanesulfinate
- Potassium bicarbonate
- Trifluoromethanesulfonyl chloride
- Trifluoromethanesulfonic acid
- Trimethylsilyl trifluoromethanesulfonate
- 4-BROMOPHENYL TRIFLUOROMETHANESULFONATE
- Bismuth(III) trifluoromethanesulfonate
- COPPER(II) TRIFLUOROMETHANESULFONATE
- Zinc trifluoromethanesulfonate
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine