Manufacturing Process
5-Acetylamino-2-acetoxybenzoyl chloride was obtained by reaction of 5acetylamino-2-acetoxy-benzoic acid with thionylchloride.
5-Acetylamino-N-butyl-2-hydroxybenzamide was produced in the result of treatment of 5-acetylamino-2-acetoxybenzoyl chloride with butylamine in the
presence of sodium hydroxide
5-Acetamino-N-(n-butyl)-2-propargyloxybenzamide was obtained by reaction of 5-acetylamino-N-butyl-2-hydroxybenzamide with propargylbromide in the presence of sodium, isopropyl alcohol and sulfuric acid.
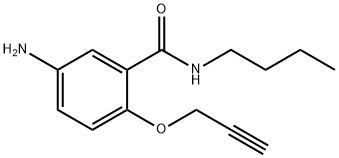
28.8 g (0.1 mole) 5-acetamino-N-(n-butyl)-2-propargyloxybenzamide in 320 ml of 4 N sulfuric acid was heated, under stirring, at 90°-95°C for 2 h. The clear solution was cooled and its pH adjusted to 1 with 1 N NaOH; after filtering, further alkali was subsequently added until a pH of 10 was obtained. At this point the product was separated by filtration and recrystallized from ethanol at 60°C to give 16.6 g (a yield of 68%) of chromatographically pure 5-amino-N-(n-hutyl)-2-propargyloxybenzamide; melting point 85°-87°C.