Description
Flunarizine is the difluorinated derivative of cinnarizine.
Thus, its preparation and therapeutic
use are identical to those of cinnarizine.
Originator
Sibelium,Janssen,W. Germany,1977
Definition
ChEBI: Flunarizine is a diarylmethane.
Manufacturing Process
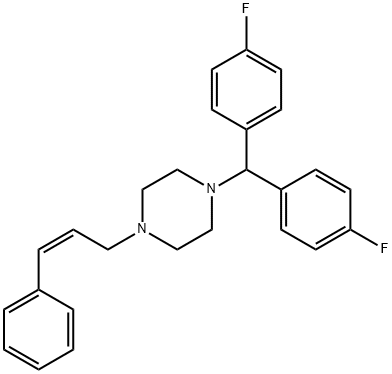
A mixture of 14.3 parts of di-(p-fluorophenyl)-chloromethane, 10.1 parts of 1-
cinnamylpiperazine, 12.7 parts of sodium carbonate, a few crystals of
potassium iodide in 200 parts of 4-methyl-2-pentanone is stirred and refluxed
for 21 hours. The reaction mixture is cooled and 50 parts of water are added.
The organic layer is separated, dried, filtered and evaporated.
The oily residue is dissolved in 480 parts of anhydrous diisopropyl ether. This
solution is boiled with activated charcoal, filtered and to the clear filtrate is
added an excess of 2-propanol, previously saturated with gaseous hydrogen
chloride. The precipitated salt is filtered off and recrystallized from a mixture
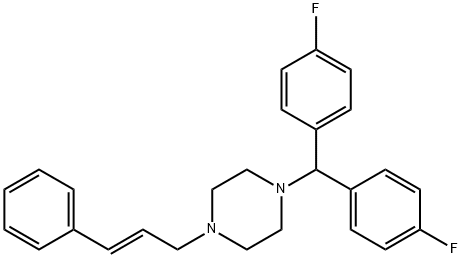
of 2-propanol and ethanol, yielding 1-cinnamyl-4-(di-p-fluorobenzhydryl)
piperazine dihydrochloride, MP 251.5°C.
Therapeutic Function
Vasodilator
World Health Organization (WHO)
Flunarizine, an antihistaminic and vasodilator agent, was
introduced into medicine in 1970. It is indicated for the treatment of central and
peripheral vascular disorders. However, its effectiveness in these conditions has
not been convincingly demonstrated, and its use has been associated with adverse
reactions involving the central nervous system, including extrapyramidal
disturbances and depression. This has led several regulatory authorities to restrict
the approved indications for products containing flunarizine.