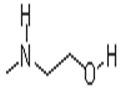
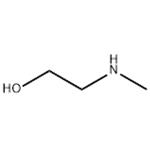
Chemical Properties
viscous colourless or light yellow liquid
Uses
2-(Methylamino)ethanol is used as an intermediate in synthetic chemistry. It finds application in various fields such as textile lubricants, polishes, detergents and in personal care products. It is also involved in electrostatic automotive coatings and in acid gas scrubbing. Further, it serves as an intermediate in polymers and pharmaceuticals. In addition to this, it is used as a solvent in the natural gas-processing industry.
Uses
Textile chemicals, pharmaceuticals.
Definition
ChEBI: An ethanolamine compound having an N-methyl substituent.
Production Methods
Monomethylethanolamine is manufactured by reacting ethylene
oxide and methylamine with external cooling.
General Description
A clear colorless liquid. Flash point 165°F. Less dense than water and soluble in water. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion. Used to make other chemicals.
Air & Water Reactions
Soluble in water.
Reactivity Profile
2-Methylaminoethanol is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. 2-Methylaminoethanol may react with oxidizing materials.
Health Hazard
Exposure can cause irritation of eyes, nose and throat.
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors and toxic gases, such as nitrogen oxides and carbon monoxide, may be formed when involved in fire.
Safety Profile
Poison by
intraperitoneal route. Moderately toxic by
ingestion and subcutaneous routes. A
corrosive irritant to skin, eyes, and mucous
membranes. Flammable when exposed to
heat, flame, or oxidizers. To fight fire, use
alcohol foam. When heated to
decomposition it emits toxic fumes such as
NOx. See also AMINES and ALCOHOLS.
Toxics Screening Level
The ITSL for 2-(Methylamino)ethanol is 38 μg/m3 based on an annual averaging time.