Synthesis
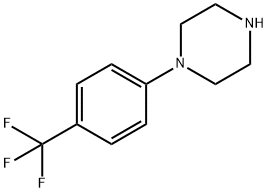
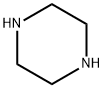
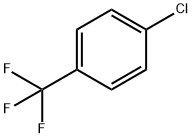
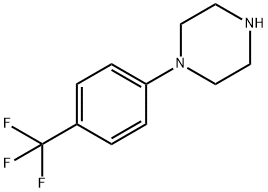
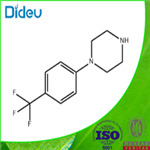
Example 4: 4-Chlorobenzotrifluoride (18.5 g, 100 mmol) and piperazine (12.9 g, 150 mmol) were dissolved in 200 mL of tri-n-butylamine and degassed by passing nitrogen for 15 minutes at room temperature. Anhydrous sodium tert-butoxide (13.5 g, 140 mmol) was then added and degassing was continued for 10 min. In another vessel, [2-(2,6-dimethoxyphenyl)phenyl]dicyclohexylphosphine (41 mg, 0.1 mmol) was stirred with (dibenzylideneacetone)palladium (20 mg, 0.025 mmol) under nitrogen protection in 5 mL of degassed tetrahydrofuran for 30 min. Afterwards, the catalyst solution was added dropwise to the main reaction flask at room temperature via a transfer needle. After dropwise addition, the reaction system was heated to an internal temperature of 110 °C and the reaction was maintained for 8 hours. Upon completion of the reaction, it was cooled to 50 °C and filtered to remove the precipitated solids. The filtrate was concentrated under reduced pressure and extracted with dilute hydrochloric acid pH 3. After separation of the aqueous phase, it was adjusted to pH 10 with sodium hydroxide solution, precipitated as a white solid, filtered and dried under reduced pressure to give N-(4-trifluoromethylphenyl)piperazine 22.1 g (96 mmol, 96% yield). The purity of the product was determined by quantitative proton NMR and confirmed to be >99%.
References
[1] Patent: US2005/250791, 2005, A1. Location in patent: Page/Page column 4
[2] Patent: KR101589584, 2016, B1. Location in patent: Paragraph 0098-0102