Description
2-Thiophenecarboxaldehyde, also known as alpha-formylthiophene or 2-thienylaldehyde, belongs to the class of organic compounds known as aryl-aldehydes. Aryl-aldehydes are compounds containing an aldehyde group directly attached to an aromatic ring. 2-Thiophenecarboxaldehyde is a sulfurous tasting compound. 2-Thiophenecarboxaldehyde has been detected, but not quantified in, asparagus (Asparagus officinalis). This could make 2-thiophenecarboxaldehyde a potential biomarker for the consumption of these foods. 2-Thiophenecarboxaldehyde is a secondary metabolite. Secondary metabolites are metabolically or physiologically non-essential metabolites that may serve a role as defense or signalling molecules. In some cases they are simply molecules that arise from the incomplete metabolism of other secondary metabolites.
Chemical Properties
clear yellow to light brown liquid
Uses
2-Thiophenecarboxaldehyde is used in the synthesis of β-aryl-β-amino acids, urea derivatives. Arylation reagent. Also used to synthesize unsaturated ketones as antiviral and cytotoxic agents.
Uses
Thiophene derivatives, introducing thenyl
group into organic compounds.
Application
2-Thenaldehyde(T2A) was originally used to produce a range of antihistamines, including methapyrilene, methaphenilene, and thenalidine. However, this usage has practically disappeared.
The anthelmintic pyrantel is an important outlet for T2A, enhanced by new formulations and the development of the medicinal use of pyrantel beyond the original veterinary market.
An important T2A derivative is the antihypertensive eprosartan (2-thiophenepropionic acid methyl ester), which acts as a selective angiotensin II receptor antagonist.
?Other pharmaceuticals containing T2A are azosemide a diuretic, and teniposide an antineoplastic.
Definition
ChEBI: 2-Thiophenecarboxaldehyde is an aldehyde that is thiophene substituted by a formyl group at position 2. It has a role as a metabolite. It is a member of thiophenes and an aldehyde.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Synthesis
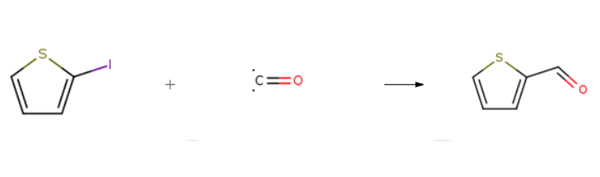
2-Thiophenecarboxaldehyde is prepared by the reaction of 2-Iodothiophene and carbon monoxide. The specific synthesis steps are as follows:
General procedure: A flask was charged with aryl iodide 1 (0.5 mmol), Pd(OAc)2 (2.4 mg, 0.01mmol), Na2CO3 (53.1 mg. 0.5 mmol), NaHCO3 (42.0 mg, 0.5 mmol), and PEG-400 (2 g) beforestandard cycles evacuation and backfilling with dry and pure carbon monoxide. Triethylsilane(162.8 μl, 1.0 mmol) was added successively. Then, the mixture was stirred at room temperaturefor the indicated time. At the end of the reaction, the reaction mixture was extracted with diethylether (3 × 10 mL). The organic phases were combined, and the volatile components wereevaporated under reduced pressure. The crude product was purified by column chromatography onsilica gel (petroleum ether / diethyl ether).
Purification Methods
Wash it with 50% HCl and distil it under reduced pressure just before use. It has UV: 234nm (hexane). The Z-oxime has m 144o, 136-138o and 142o (H2O). [Beilstein