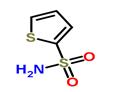
Synthesis
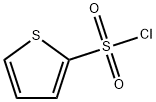
General procedure for the synthesis of 2-thiophenesulfonamide from 2-thiophenesulfonyl chloride:
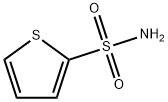
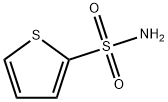
Example: preparation of 2-thiophenesulfonamide
2-Thiophenesulfonyl chloride (0.5 g, 2.74 mmol) was added to 25% ammonium hydroxide solution (5 mL, 33.45 mmol), refluxed and stirred at 50°C for 15 hours. Upon completion of the reaction, the reaction mixture was filtered while hot and the residue was washed with boiling aqueous 25% ammonium hydroxide solution. Subsequently, the ammonium hydroxide solution was removed by vacuum distillation until there was no ammonia odor. Eventually, 2-thiophenesulfonamide was recrystallized from water to give a white solid (0.328 g, 77% yield).
Product Characterization:
Melting point: 145-146°C;
IR spectrum (KBr, cm?1): 3290 (N-H stretching vibration), 3224 (N-H stretching vibration);
NMR hydrogen spectrum (400 MHz, DMSO-d6, δ): 7.15 (1H, dd, J = 5.0, 3.7 Hz, C(4)H), 7.55 (1H, dd, J = 3.7, 1.4 Hz, C(3)H), 7.66 (2H, s, NH2), 7.85 (1H, dd, J = 5.0, 1.4 Hz, C(5)H);
NMR carbon spectrum (100 MHz, DMSO-d6, δ): 127.3, 130.0, 131.1, 145.7;
Mass spectrum (ESI?): m/z 162 ([M-H]? , 100%);
High-resolution mass spectrum (ESI?): C4H4NO2S2 ([M-H]?) Calculated value 161.9689, measured value 161.9695.
References
[1] Journal of the American Chemical Society, 2010, vol. 132, # 10, p. 3238 - 3239
[2] Patent: WO2011/55142, 2011, A2. Location in patent: Page/Page column 47
[3] Phosphorus and Sulfur and the Related Elements, 1981, vol. 10, p. 111 - 120
[4] Phosphorus and Sulfur and the Related Elements, 1980, vol. 8, p. 197 - 200
[5] Justus Liebigs Annalen der Chemie, 1933, vol. 501, p. 174,182