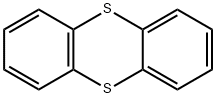
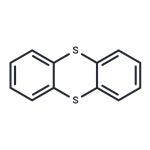
Chemical Properties
White to off-white solid
Uses
Thianthrene has been used to study aqueous solubilities of several solid nitrogen-, sulfur- and oxygen-containing heterocyclic derivatives of anthracene, phenanthrene and fluorene. It has been used in determination of partition coefficients of several sulfur-containing aromatics in 1-hexyl-3-methylimidazolium
bis(trifluoromethylsulfonyl)imide and supercritical carbon dioxide.
Definition
ChEBI: Thianthrene is the organosulfur heterocyclic compound that is the parent compound of the thianthrenes, a tricyclic structure comprising two benzene rings fused to the b and e sides of 1,4-dithin. It is a mancude organic heterotricyclic parent, an organosulfur heterocyclic compound and a member of thianthrenes.
Preparation
Thianthrene was first synthesized by John Stenhouse by dry distillation of sodium benzenesulfonate. Thianthrene can be prepared by treating benzene with disulfur dichloride in the presence of aluminium chloride.
Synthesis Reference(s)
The Journal of Organic Chemistry, 31, p. 4071, 1966
DOI: 10.1021/jo01350a045
General Description
Thianthrene undergoes liquid phase
tert-butylation in the presence of large pore zeolites and mesoporous aluminosilicates catalyst to yield
tert-butyl derivatives. Thianthrene on oxidation in the presence of hydrogen peroxide and ligninase as catalyst from
Phanerochaete chrysosporium yields thianthrene monosulfoxide.
Purification Methods
thianthrene from Me2CO (charcoal), AcOH or EtOH. It sublimes in a vacuum. [Beilstein 19 H 45, 19 I 619, 19 II 34, 19 III/IV 347, 19/2 V 49.]
References
[1] STENHOUSE J. Ueber die Producte der trockenen Destillation der sulfobenzolsauren Salze[J]. European Journal of Organic Chemistry, 1869, 149 2: 247-255. DOI:
10.1002/jlac.18691490216.
[2] BOGDAN BODUSZEK Henry J S. Preparation of solid thianthrene cation radical tetrafluoroborate[J]. The Journal of Organic Chemistry, 1988, 53 21: 5142-5143. DOI:
10.1021/jo00256a042.
[3] XIAO-YUE CHEN. Thianthrene Radical Cation as a Transient SET Mediator: Photoinduced Thiocyanation and Selenocyanation of Arylthianthrenium Salts?[J]. Chinese Journal of Chemistry, 2023, 41 16: 1979-1986. DOI:
10.1002/cjoc.202300188.
[4] RANDY A. JOHNSON Lon J M. Synthesis and Characterization of Thianthrene-Based Polyamides[J]. Macromolecules, 1995, 28 1: 79-85. DOI:
10.1021/ma00105a009.