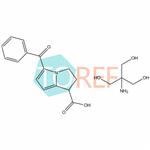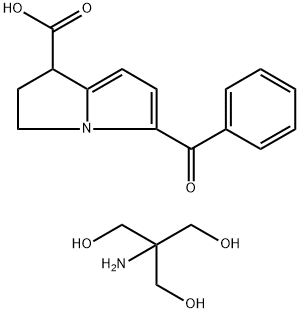
Ketorolac tromethamine
- Product NameKetorolac tromethamine
- CAS74103-07-4
- CBNumberCB2427540
- MFC19H24N2O6
- MW376.41
- MDL NumberMFCD00887595
- MOL File74103-07-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 160-161 C |
| storage temp. | 2-8°C |
| solubility | H2O: 15 mg/mL stable at least one month at −20 °C., soluble |
| form | crystalline |
| color | White to Light yellow |
| biological source | synthetic (organic) |
| Water Solubility | H2O: soluble 15mg/mL, clear, colorless to faintly yellow (stable at least one month at -20 °C.) |
| λmax | 322nm(MeOH)(lit.) |
| Merck | 14,5306 |
| Stability | Hygroscopic |
| InChI | InChI=1S/C15H13NO3.C4H11NO3/c17-14(10-4-2-1-3-5-10)13-7-6-12-11(15(18)19)8-9-16(12)13;5-4(1-6,2-7)3-8/h1-7,11H,8-9H2,(H,18,19);6-8H,1-3,5H2 |
| InChIKey | BWHLPLXXIDYSNW-UHFFFAOYSA-N |
| SMILES | C(N)(CO)(CO)CO.C(C1C=CC=CC=1)(C1=CC=C2C(C(=O)O)CCN12)=O |
| CAS DataBase Reference | 74103-07-4(CAS DataBase Reference) |
| FDA UNII | 4EVE5946BQ |
| NCI Drug Dictionary | ketorolac tromethamine |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301-H315-H319-H335 | |||||||||
| Precautionary statements | P301+P310+P330-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-36/37/38-23/24/25 | |||||||||
| Safety Statements | 26-45-36/37/39 | |||||||||
| RIDADR | UN 2811 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UY7759900 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 2933995500 | |||||||||
| NFPA 704: |
|