Chemical properties
It is crystallized in ethyl acetate - ether with a melting point of 160~161 degrees centigrade.The maximum absorption of UV (methanol):245,312m (epsilon 7080,17400). PKa3.49 + 0.02.Acute toxic LD50 mice (mg/kg): about 200 of oral administration. .(+) - configuration: crystallized from hexane ethyl acetate with the melting point of 174 degrees centigrade;The melting point is 154 156 degrees centigrade. [α]D+173° (C=1, methanol).(-) - configuration: crystallizzed from hexane ethyl acetate with melting point at 169~170 degrees centigrade; melting point is 153~155 degrees centigrade. [α]D-176° (C=1, methanol).
Pharmacological action
Ketorolac is also called ketorolac, acular and Ketorolac Tromethamine. It is a non steroid analgesic and anti-inflammatory drug, a derivative of pyrrolidone.The chemical structure and pharmacological action is similar to Tolectin, Zoaesthetic acid and Indometacin. By inhibiting cyclooxygenase, it inhibits the synthesis and release of prostaglandins, and produces anti-inflammatory effects. It can reduce the temperature induced by heat source, which is related to the inhibition of the synthesis of prostaglandin in the central nervous system.It has strong analgesic and moderate anti-inflammatory antipyretic and inhibition of platelet aggregation, and has no inhibition of respiration and addiction. In animal experiments, the analgesic effect is stronger than aspirin, indomethacin and naproxen.It is equal to or better than the anti-inflammatory effect of naproxen and indomethacin, phenylbutazone,Its antipyretic effect on rats are stronger than aspirin and phenylbutazone and the same with indomethacin and naproxen.It inhibits the platelet aggregation induced by arachidonic acid and collagen.But it does not inhibit the induction of adenosine diphosphate(ADP) ..This product is quickly and completely absorbed after the intramuscular injection, and is almost completely absorbed after oral administration.Food can slow down the absorption speed, but it does not affect the degree of absorption, and the bioavailability is 80% ~ 100%. 10 minutes after intramuscular injection of 30mg, it usually relieves the pain obviously. After 50 minutes, the peak of plasma concentration is up to 2.2 g/mL.After 30~60 minutes of oral administration, the pain is obviously relieved and the concentration of plasma is peak at 1.5 to 4 hours.The plasma half-life of young people is about 5.3 hours, and for the elderly it is about 7 hours, and the analgesic effect could be maintained for 6~8 hours. 91.4% will be excreted from the urinary tract, the rest will be excreted from the excrement.In patients with renal insufficiency, the total plasma clearance rate decreases and the half-life prolongs, so the dosage should be reduced.
Clinical application: ketorolac is mainly used for short-term pain treatment including postoperative pain (such as the abdomen, chest, Urology, gynecology, Department of Stomatology, orthopedic surgery and pain) as well as the acute skeletal muscle pain caused by various causes, such as sprain, dislocation, fracture and soft tissue injury, and other pain caused by other diseases, such as postpartum pain, acute renal colic, toothache, sciatica, late cancer pain, wound pain, biliary colic, etc.. It can be used as a substitute for morphine and pethidine.
Chemical Properties
Light yellow solid
Uses
Ketorolac-d5 is a labeled analogue of Ketorolac, a Prostaglandin biosynthesis inhibitor. Analgesic; anti-inflammatory.
Uses
prostaglandin F2a analogue
Indications
Ketorolac (Toradol), an NSAID chemically related
to indomethacin and tolmetin, is mainly used as an analgesic,
not for the treatment of inflammatory disease. It
is available in oral, parenteral, and topical formulations.
Definition
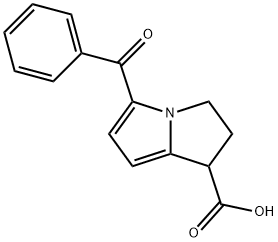
ChEBI: Ketorolac is a racemate comprising equimolar amounts of (R)-(+)- and (S)-(-)-5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid. While only the (S)-(-) enantiomer is a COX1 and COX2 inhibitor, the (R)-(+) enantiomer exhibits potent analgesic activity. A non-steroidal anti-inflammatory drug, ketorolac is mainly used (generally as the tromethamine salt) for its potent analgesic properties in the short-term management of post-operative pain, and in eye drops to relieve the ocular itching associated with seasonal allergic conjunctivitis. It was withdrawn from the market in many countries in 1993 following association with haemorrhage and renal failure. It has a role as a cyclooxygenase 2 inhibitor, a cyclooxygenase 1 inhibitor, a non-steroidal anti-inflammatory drug and an analgesic. It contains a (R)-ketorolac and a (S)-ketorolac. It is a conjugate acid of a ketorolac(1-).
brand name
Acular (Allergan); Toradol (Roche).
World Health Organization (WHO)
Ketorolac is a nonsteroidal anti-inflammatory agent used in the
management of moderate to severe acute post-operative pain. It remains on the
market in many countries with restrictions on its use.
Biological Functions
Ketorolac (Toradol) is an NSAID with very mild antiinflammatory
and antipyretic activity. It is a potent analgesic
for postoperative pain. Its efficacy is equivalent to
that of low doses of morphine in the control of pain. For
this reason it is often combined with opioids to reduce
opioid dose and related side effects while providing adequate
pain relief. It is also used to replace the opioids in
some patients with opioid sensitivity. The mechanism of
action of ketorolac involves the inhibition of COX and
decreased formation of prostaglandins. However, some
evidence exists that ketorolac may stimulate the release
of endogenous opioids as a part of its analgesic activity.
General Description
Ketorolac tromethamine (Toradol), marketed as a mixture of(R)- and (S)-ketorolac enantiomers, is a potent NSAID analgesicindicated for the treatment of moderately severe, acutepain. It should be noted that the pharmacokinetic dispositionof ketorolac in humans is subject to marked enantioselectivity.Thus, it is important to monitor the individual blood levelsso an accurate assessment of its therapeutic action can bemade correctly. However, it should be noted that, beingone of the conventional NSAIDs with highest risk of GIcomplications, its administration should not exceed 5 days.
Clinical Use
Ketorolac is a nonsteroidal
anti-inflammatory drug mainly used
for the treatment of moderate to severe postoperative
pain. Ketorolac shows a balanced inhibition
of COX-1 and COX-2 in cultured human
cells . Ketorolac is mostly used as
the tromethamine salt. Due to a number of severe
side effects including gastrointestinal disturbances,
impairment of liver functions, renal
failures, skin irritations, and other hypersensitivity
reaction it has been withdrawn in many
countries.
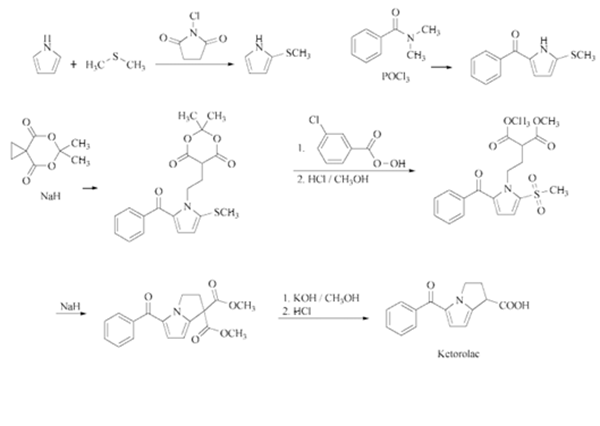
Synthesis
Benzoylation of 2-methylthiopyrrole
with N,N-dimethylbenzamide in the
presence of POCl3 in refluxing CH2Cl2 gives
5-benzoyl-2-methylthiopyrrole, which is condensed
with spiro-5,7-dioxa-6,6-dimethyloctane-
4,8-dione by means of NaH in DMF.
Oxidation of this product with m-chloroperbenzoic
acid in CH2Cl2 affords the sulfone, which
is submitted to methanolysis with methanol and
HCl giving 1-(3,3-dimethoxycarbonylpropyl)-
2-methanesulfonyl-5-benzoylpyrrole. Cyclization with NaH inDMFyields dimethyl-5-benzoyl-
1,2-dihydro-3H-pyrrolopyrrole-1,1-
dicarboxylate, which is finally hydrolyzed and
decarboxylated withKOHin refluxing methanol
.