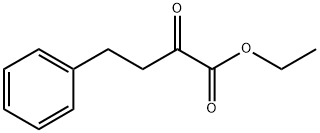
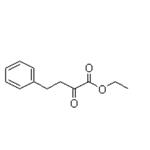
synthesis
Potassium tert-butoxide (4.3 g, 38.34 mmol) was added in 1 portion to a solution of the diester (8 g, 25.56 mmol) in toluene (80 mL) at 0 °C. After being stirred at the same temperature for 30 min, the reaction mixture was kept at room temperature overnight. Water (100 mL) was added, the phases were separated, and the aqueous phase was extracted with EtOAc (3 × 100 mL). The combined organic extracts were dried and evaporated. The residue was purifified by flflash chromatography eluting with EtOAc:light petroleum (1:9) to give 5.6 g (78%) of the keto-ester as an orange oil.
Chemical Properties
light yellow oily liquid
Uses
Ethyl 2-oxo-4-phenylbutyrate may be used in the synthesis of ethyl (
R)-2-hydroxy-4-phenylbutyrate, an important chiral precursor for angiotensin-converting enzyme (ACE) inhibitor.
General Description
Ethyl 2-oxo-4-phenylbutyrate is an aliphatic α-ketoester. Bioreduction of ethyl 2-oxo-4-phenylbutyrate is reported to yield ethyl (
R)-2-hydroxy-4-phenylbutanoate. The effect of ionic liquid on the asymmetric reduction of ethyl 2-oxo-4-phenylbutyrate by
Saccharomyces cerevisiae has been reported. Asymmetric reduction of ethyl 2-oxo-4-phenylbutyrate using a bacterial reductase is reported. Enantioselective hydrogenation of ethyl 2-oxo-4-phenylbutyrate using homogeneous Rh-diphosphine and heterogeneous Pt/Al
2O
3-cinchona catalysts has been reported.