Originator
Syncillin, Bristol, US ,1959
Definition
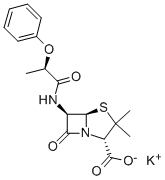
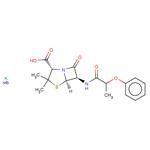
ChEBI: Phenethicillin potassium is an organic potassium salt. It contains a phenethicillin(1-).
Application
Phenethicillin was the first member of the semisynthetic penicillin class of antibiotics to be introduced in clinics. This drug was synthesized in 1960 by Bristol-Myers in collaboration with Beecham Research Laboratories starting with 6-aminopenicillanic acid. It is as stable against gastric acid as phenoxymethylpenicillin and is used orally for therapy of gram-positive and Neisseria infections.
Manufacturing Process
Triethylamine (1.5 ml) was added to a cold solution (10°C) of αphenoxypropionic acid (1.66 g, 0.01 mol) in 15 ml of pure dioxane, with stirring and cooling to 5°C to 10°C while isobutyl chloroformate (1.36 g, 0.01 mol) in 5 ml of dioxane was added dropwise. Then the mixture was stirred for ten minutes at 5°C to 8°C. A solution of 6-amino-penicillanic acid (2.16 g, 0.01 mol) in 15 ml of water and 2 ml of triethylamine was then added dropwise while the temperature was maintained below 10°C. The resulting mixture was stirred in the cold for 15 minutes then at room temperature for 30 minutes, diluted with 30 ml of cold water and extracted with ether which was discarded. The cold aqueous solution was then covered with 75 ml of ether and acidified to pH 2 with 5 N H2SO4. After shaking, the ether layer containing the product 6-(α-phenoxypropionamido)penicillanic acid, was dried for ten minutes over anhydrous sodium sulfate and filtered. Addition of 6 ml of dry n-butanol containing 0.373 g/ml of potassium 2-ethylhexanoate precipitated the potassium salt of the product as a colorless oil which crystallized on stirring and scratching and was collected, dried in vacuo and found to weigh 2.75 g, to melt at 217°C to 219°C.
brand name
Chemipen
(Bristol-Myers Squibb); Syncillin (Bristol-Myers
Squibb) [Name previously used: Phenethicillin.].
Therapeutic Function
Antibacterial
Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic fumes of K2O, SOx, and NOx.