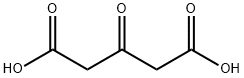
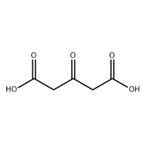
Chemical Properties
white to off-white powder
Physical properties
Acetonedicarboxylic
acid forms colorless crystals that are readily soluble in water and ethanol, and sparingly soluble in
trichloromethane and diethyl ether. It melts with
decomposition at 138 ℃.
Uses
β-Ketoglutaric Acid is used in the synthesis of benzodiazepine derivatives. Also used in the synthesis of orally available hepatitis C virus polymerase inhibitors.
Uses
Acetone-1,3-dicarboxylic acid is used as a building block in organic chemistry. It is also used in the synthesis of hetrocyclic rings and in the Weiss-Cook reaction, which involves the preparation of cis-bicyclo[3.3.0]octane-3,7-dione by reacting with diacyl (1,2 ketone). Its presence in human urine is used as a diagnostic test for the overgrowth of harmful gut flora such as Candida albicans.
Definition
ChEBI: 3-Oxoglutaric acid is an oxo carboxylic acid.
Production Methods
Acetonedicarboxylic acid is
produced from citric acid [77-92-9] by many
industrial processes that differ only slightly. Citric acid is treated with oleum and
reacts to yield acetonedicarboxylic acid via decarbonylation and dehydration.
Other possible production methods include
reaction of acetone with carbon dioxide, oxidation of citric acid with chlorosulfuric
acid, or reaction of ketene with phosgene.
Flammability and Explosibility
Non flammable
Purification Methods
Crystallise it from ethyl acetate and store it over P2O5. It decarboxylates in hot water. [Beilstein 3 IV 1816.]