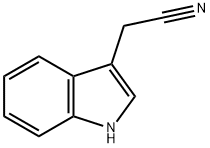
Chemical Properties
clear yellow-brown to brown liquid after melting
Uses
Reactant for preparation of:
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Histone deacetylase inhibitors
- Potential kinase inhibitors
- Kv7/KCNQ potassium channel activators
- Kinesin-Specific MKLP-2 Inhibitor
- Pesticides
- Potential PET cancer imaging agents
- Agonists of the Farnesoid X Receptor (FXR) as atherosclerosis treatment
- Butyrylcholinesterase inhibitors
- Necroptosis inhibitors
Definition
ChEBI: A nitrile that is acetonitrile where one of the methyl hydrogens is substituted by a 1H-indol-3-yl group.
Synthesis Reference(s)
Canadian Journal of Chemistry, 39, p. 1340, 1961
DOI: 10.1139/v61-169Journal of the American Chemical Society, 75, p. 3589, 1953
DOI: 10.1021/ja01110a506
General Description
3-Indoleacetonitrile (Indolylacetonitrile) is a light-induced auxin-inhibitory substance that is isolated from light-grown cabbage (
Brassica olearea L.) shoots. It inhibits the biofilm formation of both
E. coli O157:H7 and
P. aeruginosa without affecting its growth.