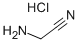Chemical Properties
White to light brown solid
Uses
Used as pharmaceutical intermediates, organic synthetic raw material transportation.
Application
Aminoacetonitrile is a useful synthetic intermediate. It is a reagent used to synthesize dipeptide nitriles as reversible and potent cathepsin S inhibitors. It can also used to prepare substituted cyclic ureas as possible HIV protease inhibitors.
Preparation
Synthesis of Aminoacetonitrile Hydrochloride by Reaction of Aminoacetonitrile with Hydrogen Chloride in Methanol: mixing the aminoacetonitrile with hydrogen chloride methanol solution, reacting at 45-50°C for 1-2 hours, cooling to below 5°C, filtering, and centrifugalizing to obtain the aminoacetonitrile hydrochloride, wherein in the hydrogen chloride methanol solution, the content of hydrogen chloride is 30-50 wt%, and the water content is less than or equal to 1%.
Preparation method of aminoacetonitrile hydrochloride
Safety Profile
An experimental
teratogen. Experimental reproductive
effects. When heated to decomposition it
emits toxic fumes of NOx and HCl.
Purification Methods
The salt recrystallises from dilute EtOH as hygroscopic leaflets. It is best to crystallise it from absolute EtOH/Et2O (1:1) and then recrystallise it from absolute EtOH. The melting point recorded ranges from 144o to 174o. The free base has b 58o/15mm with partial decomposition. [Klages J Prakt Chem [2] 65 189 1902, Mange J Am Chem Soc 56 2197 1934, Goldberg & Kelly J Chem Soc 1371 1947, Beilstein 4 H 344, 4 I 468, 4 II 783, 4 III 1120, 4 IV 2363.]