Uses
Reactant for preparation of indolyl ethanones as indoleamine 2,3-dioxygenase inhibitors 1 Reactant for preparation of histamine H3 receptor inverse agonists 2 Reactant for preparation of PPAR-γ binding agents with potential application to the treatment of osteoporosis 3 Reactant for preparation of Nonpeptide glycoprotein IIB/IIIA inhibitors 4
Synthesis
1.1) 80 ml of sulfuric acid was dissolved in 450 mL of ethanol and cooled to 0C;
1.2) 359 mmol (90 g) of 4-benzyloxyphenylhydrazine hydrochloride was added to the mixture of 1.1) to form a suspension;
1.3) 43.9 mL of ethyl pyruvate was added to 90 mL of anhydrous ethanol;
1.4) the suspension of step 1.2) was added to step 1.3), magnetically stirred for 2h, warmed up to 45C, and held to react for 16h;
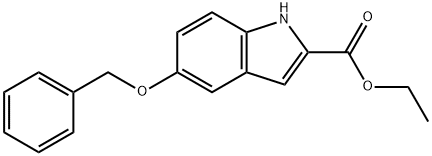
1.5) the solution of step 1.4) was cooled to room temperature, 200mL of ethanol was added, filtered, and the solids were washed with ethanol, cyclohexane, and water in turn, dried, and yielded compound 3, i.e., 5-benzyloxyindole-2-carboxylic acid ethyl ester, in 72% yield ( 76.3g).