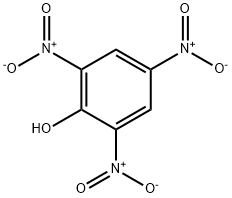
PICRIC ACID
- Product NamePICRIC ACID
- CAS88-89-1
- CBNumberCB1195194
- MFC6H3N3O7
- MW229.1
- EINECS201-865-9
- MDL NumberMFCD00007102
- MOL File88-89-1.mol
Chemical Properties
| Melting point | 122-123 °C (dried material, Lit. Merck Index 12th Ed.)(lit.) |
| Boiling point | 300℃ |
| Density | 1.00 g/mL at 20 °C |
| vapor density | 7.9 (vs air) |
| vapor pressure | 1 mm Hg ( 195 °C) |
| refractive index | 1.7630 (estimate) |
| Flash point | 150℃ |
| storage temp. | Store at RT. |
| solubility | alcohol: soluble1 (g/12 mL)(lit.) |
| pka | 0.38(at 25℃) |
| form | solution (saturated aqueous) |
| Colour Index | 10305 |
| Specific Gravity | 1.005 |
| color | yellow solution |
| PH Range | 0.2(colourless)-1(yellow) |
| explosive limit | 0.01% |
| Water Solubility | (mg/L): 66,670 at 100 °C (quoted, Windholz et al., 1983) 14,000 at 20 °C, 68,000 at 100 °C (quoted, Verschueren, 1983) |
| λmax | 354nm |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H201-H301+H311+H331 | |||||||||
| Precautionary statements | P210-P230-P250-P280-P301+P310-P370+P372+P380+P373-P503 | |||||||||
| Hazard Codes | F,T,E,Xn | |||||||||
| Risk Statements | 1-4-11-23/24/25-2-36-20/21/22-3 | |||||||||
| Safety Statements | 35-45-37-28-36/37-36-26-16-25 | |||||||||
| RIDADR | UN 1344 4.1/PG 1 | |||||||||
| OEB | D | |||||||||
| OEL | TWA: 0.1 mg/m3, STEL: 0.3 mg/m3 [skin] | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | TJ7875000 | |||||||||
| HazardClass | 4.1 | |||||||||
| PackingGroup | I | |||||||||
| Hazardous Substances Data | 88-89-1(Hazardous Substances Data) | |||||||||
| Toxicity | LC50 (48-h) for red killifish 513 mg/L (Yoshioka et al., 1986). | |||||||||
| IDLA | 75 mg/m3 | |||||||||
| NFPA 704: |
|