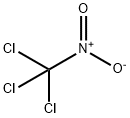
Trichloronitromethane
- Product NameTrichloronitromethane
- CAS76-06-2
- CBNumberCB4377500
- MFCCl3NO2
- MW164.38
- EINECS200-930-9
- MDL NumberMFCD00041878
- MOL File76-06-2.mol
Chemical Properties
| Melting point | -64°; mp -69.2° (corr) |
| Boiling point | 112 °C |
| Density | 1.657 |
| vapor pressure | 18.3 at 20 °C (Meister, 1988) |
| refractive index | 1.461 |
| storage temp. | 0-6°C |
| solubility | Miscible with acetone, benzene, carbon disulfide, carbon tetrachloride, ether, and methanol (Worthing and Hance, 1991) |
| form | Oily Liquid |
| Water Solubility | 2,270 mg/L at 0 °C (Gunther et al., 1968) 1.621 g/L at 25 °C (quoted, Windholz et al., 1983) |
| Merck | 13,2175 |
| BRN | 1756135 |
| Henry's Law Constant | 2.44 (static headspace-GC, Welke et al., 1998) |
| Dielectric constant | 7.3200000000000003 |
| Exposure limits | NIOSH REL: TWA 0.1 ppm, IDLH 2 ppm; OSHA PEL: TWA 0.1 ppm; ACGIH TLV: TWA 0.1 ppm, STEL 0.3 ppm. |
| Stability | Stable. May decompose violently if heated. Large volumes of this chemical may be shock-sensitive. Reacts violently with sodium methoxide, propargyl bromide and aniline. Incompatible with 3-bromopropyne, strong oxidizers, plastics, rubber, iron, zinc and other light metals. |
| LogP | 2.090 |
| CAS DataBase Reference | 76-06-2(CAS DataBase Reference) |
| EWG's Food Scores | 2-5 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H319-H330-H335 | |||||||||
| Precautionary statements | P260-P284-P305+P351+P338-P310 | |||||||||
| Hazard Codes | T+ | |||||||||
| Risk Statements | 22-26-36/37/38 | |||||||||
| Safety Statements | 36/37-38-45 | |||||||||
| RIDADR | UN 1580 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.7 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | PB6300000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29049030 | |||||||||
| Hazardous Substances Data | 76-06-2(Hazardous Substances Data) | |||||||||
| Toxicity | Acute oral LD50 for rats 250 mg/kg (RTECS, 1985). | |||||||||
| IDLA | 2 ppm | |||||||||
| NFPA 704: |
|