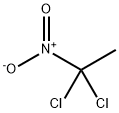
1,1-DICHLORO-1-NITROETHANE
- Product Name1,1-DICHLORO-1-NITROETHANE
- CAS594-72-9
- CBNumberCB1192638
- MFC2H3Cl2NO2
- MW143.96
- EINECS209-854-0
- MDL NumberMFCD00128006
- MOL File594-72-9.mol
- MSDS FileSDS
Chemical Properties
| Boiling point | 123.5°C (estimate) |
| Density | 1.2738 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| form | Colorless liquid |
| Water Solubility | 4.975g/L(20 ºC) |
| Dielectric constant | 16.300000000000001 |
| CAS DataBase Reference | 594-72-9 |
| EWG's Food Scores | 1 |
| FDA UNII | 1GV6MXB8TD |
| EPA Substance Registry System | 1,1-Dichloro-1-nitroethane (594-72-9) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301-H311-H331 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P280-P302+P352-P312-P322-P361-P363-P405-P501-P261-P271-P304+P340-P311-P321-P403+P233-P405-P501 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 23/24/25 | |||||||||
| Safety Statements | 26-45 | |||||||||
| RIDADR | 2650 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 2 ppm (10 mg/m3) | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| Hazardous Substances Data | 594-72-9(Hazardous Substances Data) | |||||||||
| IDLA | 25 ppm | |||||||||
| NFPA 704: |
|