Manufacturing Process
A mixture of 3-bromoadamantane-1-carboxylic acid (5.8 g), acetonitrile (45
ml) and concentrated sulphuric acid (9 ml) was refluxed for 20 h. After
cooling, the mixture was poured into water (250 ml), and the resulting
suspension concentrated in vacuo to remove most of the acetonitrile. Aqueous
sodium hydroxide (33%) was added until the pH was 4.0 (about 24 ml). The
precipitate was filtered off, washed with water, and dried to yield 4.4 g of the
3-acetaminoadamantane-1-carboxylic acid, melting point 254°-258°C (two
recrystallizations from methanol-aceto nitrile).
A solution of 3-acetamino-adamantane-1-carboxylic acid (3.0 g) in 4 N sodium
hydroxide (40 ml) was refluxed for 5 h. After cooling, the pH of the solution
was adjusted to 7 with acetic acid. The crystalline precipitate was filtered off,
washed with ethanol and dried to yield 2.20 g of the desired compound,
melting point over 330°C. In order to purify the compound, 2.0 g of it was
suspended in water (10 ml), 4 N NaOH (2 ml) was added, and the resulting
solution filtered through a filter aid known under the registered trademark
'Dicalite'. The filtrate was adjusted to a pH of 6.5 with acetic acid. The
resulting crystalline precipitate was filtered off, washed with a little water
followed by alcohol, and dried to yield 1.55 g of pure 3-amino-adamantane-1-
carboxylic acid.
3-Aminoadamantane-1-carboxylic acid (1.0 g) was refluxed with thionyl
chloride (4.6 ml) for 1 h. Excess of thionyl chloride was removed in vacuo.
The residue was dissolved in benzene (3 ml), and the resulting solution
evaporated under reduced pressure to remove traces of thionyl chloride. This
process was repeated to leave 1.3 g of a 3-thionyliminoadamantane-1-
carboxylic acid chloride, almost colourless.
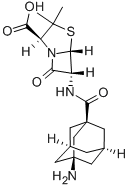
500.0 mg of 3-thionyliminoadamantane-1-carboxylic acid chloride were
dissolved in dry acetone (8 ml), and the resulting solution was added during
20 min at room temperature with stirring, to a suspension of 6-
aminopenicillanic acid (520.0 mg) in 50% aqueous acetone (20 ml),
previously adjusted to a pH of 7.0 with triethylamine. During the process, a
pH value of 7.0 was maintained by the addition of a 1 N solution of
triethylamine in 50% aqueous acetone from an automatic titrator. At the end
of the reaction, a clear solution was obtained. Acetone was removed in vacuo,
and 70% of the theoretical amount of 3-amino-adamantyl-1-penicillin was
formed.
The aqueous solution was concentrated in vacuo to a volume of 4 ml. Addition
of acetone (40 ml) gave an oily precipitate which after decanting and addition
of fresh acetone gave 900.0 mg of a semicrystalline solid which contained
50% of the theoretical amount of 3-aminoadamantyl-1-penicillin.