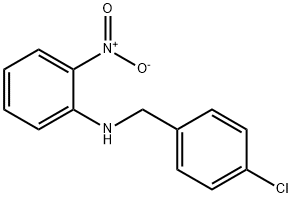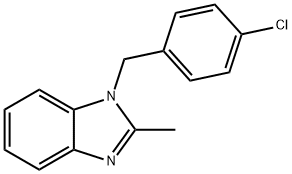
Chlormidazole
- Product NameChlormidazole
- CAS3689-76-7
- CBNumberCB0892977
- MFC15H13ClN2
- MW256.73
- EINECS222-998-9
- MOL File3689-76-7.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 67.5°C |
| Boiling point | bp12 240-242° |
| Density | 1.1578 (rough estimate) |
| refractive index | 1.5749 (estimate) |
| pka | 5.41±0.10(Predicted) |
| FDA UNII | 8IKK64FJVX |
| ATC code | D01AC04 |